- Home
- News
- Spotlight on Science
- Better cartilage...
Better cartilage imaging by phase-contrast computed tomography
16-08-2010
Phase-contrast X-ray imaging provides substantially enhanced contrast resolution for soft-tissues compared to conventional absorption radiography. Here, researchers demonstrate that phase contrast computed tomography can depict structural properties of cartilage matrix in an excised human sample. The level of detail within the tomographic images is sufficient to differentiate osteoarthritic and intact cartilage matrixes.
Share
Quasi-coherent X-rays enable visualisation of the architecture of articular cartilage, which is invisible in conventional clinical radiographs. A novel X-ray imaging approach, Phase Contrast Imaging (PCI), has been applied to study the fine structure of human cartilage under normal and pathological conditions. The experiments were performed at the ESRF Biomedical beamline (ID17). A multidisciplinary team of physicists and radiologists from the Ludwig Maximilians University (Munich, Germany) and the ESRF utilised a particular PCI method, analyser-based imaging, ABI [1]. ABI exploits the properties of X-ray diffraction using a high quality crystal to generate images where contrast is due to a combination of X-ray absorption, refraction and ultra-small angle scattering. This method permits image contrast to be improved over conventional radiography.
This research has been carried out within the German excellence cluster “Munich-centre for Advanced Photonics” (MAP). Final goals of the project are to demonstrate the applicability and the potential of PCI in selected medical diagnostic areas where currently available X-ray absorption-based imaging tools have limited diagnostic performance, and to transfer the new imaging approach to a clinical environment by using the compact high brilliance X-ray source that MAP scientists are presently developing in Munich [2].
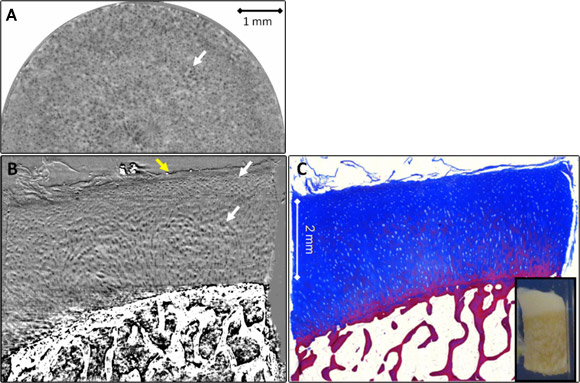
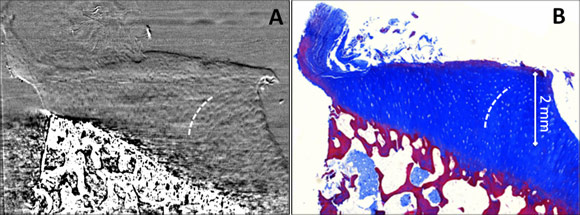
Phase contrast analyser-based tomograms (ABI-CT) show excellent depiction of the complete cartilage volume and of its 3D architecture in all samples imaged in this study. In Figures 1 and 2 reconstructed ABI-CT slices of a normal and an osteoarthritic sample are presented. The edges and interfaces between cartilage and calcified cartilage/subchondral bone and between the formalin and the cartilage surface (as indicated by the yellow arrow) are clearly discernible. These can be attributed to refraction of the beam occurring at the edges between tissues with different refraction indexes. In the ABI images low intensity oval shaped structures with an extent from 10–40 µm are detectable within the cartilage matrix. Comparison with the corresponding histology sections (Figures 1C, 2B) identifies these structures to be chondrocytes and/or chondrons (the synthetically active elements in cartilage). In all ABI data the tide mark (i.e. the interface between calcified and uncalcified cartilage), the subchondral bone plate (calcified cartilage + solid subchondral bone proper) and trabecular detail from within the spongiform bone can be observed. In particular, Figure 1A displays the above-mentioned dark oval dots (arrow), chondrocytes, against the brighter background of the cartilage matrix in an axial slice approximately at 75% depth from the surface (at the level of the radial zone). We can see that chondrocytes are aligned perpendicular to the tide mark/surface, thus their cross sections in axial slices appear as circles (Figure 1A). Coronal reformats in both, ABI (Figure 1B) and histology (Figure 1C), impressively demonstrate the typical pattern of chondrocyte alignment and distribution across the cartilage. The central portions of Figure 1B display Benninghoff’s ‘arch’ or ‘arcade’-like alignment of the chondrocytes/chondrons (i.e. clusters of chondrocytes): they initially rise vertically upwards from the tide mark but soon diverge laterally when approaching the cartilage surface following a curved trajectory. Figure 2 shows an osteoarthritic cartilage-on-bone sample. Areas of intact cartilage surface (smooth-appearance, right and mid portion of sample) alternate with subtle (right half) and distinct (left half of sample) surface erosion/defects (Figures 2A,B). The differences between the two groups of samples (normal and pathological) were clearly determined during blind trials using two independent observers in a comparison based on histopathological parameters of the ABI-CT images. A distinct zonal pattern in the cartilage matrix could consistently be visualised. The osteoarthritic samples showed significantly lower chondrocyte distribution homogeneity, less chondrocyte alignment, lower height of tangential, transitional, and radial zones and a higher prevalence of superficial cartilage damage.
The results of this proof-of-concept study indicate that the phase contrast ABI-CT technique is capable of providing soft-tissue contrast at a histopathologic level, useful to differentiate osteoarthritic and healthy cartilage samples. We were able to show that fine structural details of the cartilage matrix as well as its degeneration in early and advanced stages of osteoarthritis can be visualised by ABI-CT in relatively large samples.
If PCI could be translated into a clinical scenario, then it might evolve into an important tool for the evaluation of osteoarthritis, especially for the early detection and monitoring of the disease. Osteoarthritis involves both cartilage and bone. Yet, to date, detailed diagnostic work-up of these two tissues is generally based on two different imaging techniques: magnetic resonance imaging (MRI) and radiography/CT, respectively. In the same scenario, given its potential of visualising bony as well as cartilage structural properties and changes in osteoarthritis [3], PCI could be an imaging tool that elegantly permits comprehensive imaging based assessment of osteoarthritis using only one modality. It may be hypothesised that the ABI technique, coupled with the volumetric analysis enabled by the tomographic modality, has the potential to become a valuable non-invasive alternative to routine histological examination in orthopaedics.
References
[1] A. Bravin, Journal of Physics D: Applied Physics 36, A24-A29 (2003).
[2] P. Coan, F. Gruener, C. Glaser, T. Schneider, A. Bravin, M. Reiser, D. Habs, Nuclear Instruments and Methods in Physics Research A 608, S44-S46 (2009).
[3] P. Coan, J. Mollenhauer, A. Wagner, C. Muehleman, A. Bravin, European Journal of Radiology 68S, S41-S48 (2008).
Principal publication and authors
P. Coan (a, b), F. Bamberg (a), P.C. Diemoz (b), A. Bravin (b), K. Timpert (a), E. Mützel (c), J. Raya (a), S. Adam-Neumair (d), M.F. Reiser (a), C. Glaser (a), Characterization of Osteoarthritic and Normal Human Patella Cartilage by Computed Tomography X-ray Phase-Contrast Imaging: A feasibility Study, Investigative Radiology 45, 437-444 (2010).
(a) Ludwig-Maximilians University, Faculty of Medicine and Institute of Clinical Radiology, Munich (Germany)
(b) ESRF
(c) Ludwig-Maximilans University, Institute of Forensic Medicine, Munich (Germany)
(d) Ludwig-Maximilians University, Institute of Anatomy, Munich (Germany)
Top image: One slice from a tomogram of a normal cartilage sample showing the surface (top layer), cartilage (middle) and subchondral bone (bottom).