- Home
- News
- Spotlight on Science
- Determining the...
Determining the state of Pt in SnO2-based sensors under working conditions
09-06-2011
The role of Pt-dopants in real SnO2-based gas sensors is unknown, especially their beneficial effect on the sensors’ intrinsically poor selectivity. A combination of innovative sample design and high-energy-resolution fluorescence-detected X-ray absorption spectroscopy at beamline ID26 reveals the distribution, chemical state and function of the Pt-dopants.
Share
There is a huge market demand for products able to detect various gases for environmental control, safety, comfort and health. Gas sensors based on semiconducting metal-oxides are very attractive candidates for the detection of flammable and toxic gases due to the low cost in production and high stability. In this field, the leading role in both academic studies and applications is still held by sensors based on SnO2. To further improve their performance (sensitivity, selectivity, stability and operation temperature), minute quantities of noble metals like Pt and Pd are added [1-3], usually during synthesis of the material to ensure the presence of the additive at the surface. Although this “doping” technique has been used since the early days of metal-oxide sensors, the way in which this additive contributes to the improved performance, how it is distributed in/on the material, its chemical state in general and how it changes during the reaction is still a matter of debate. So far only studies before and after the sensing reactions or on idealised materials (with much higher Pt content than in real sensors) were reported.
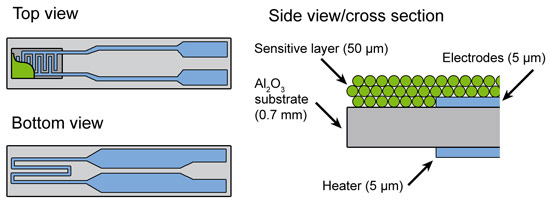
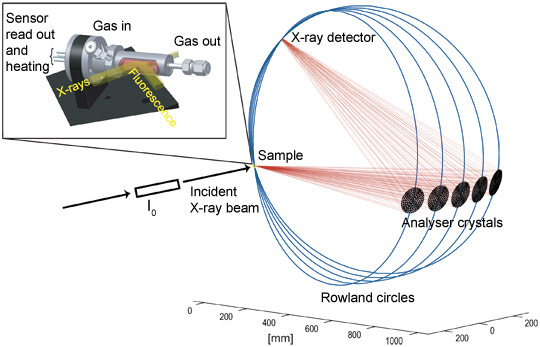
The experimental requirements are very challenging for common sensor devices because metallic Pt is also used in the electrodes and in the heater where the Pt concentration is higher by more than a factor 1000 compared to the 0.2 wt%Pt:SnO2 sensor (Figure 1). Therefore a new sensor device was designed where Pt in the heater was replaced by a Pd/Ag alloy showing similar electrical properties, while gold was used instead of Pt for the electrodes. However, the gold fluorescence energetically is very close to that of Pt, therefore in standard fluorescence detected absorption experiments the signal is dominated by the Au contribution. To differentiate between the gold and platinum fluorescence lines and to detect the small amount of Pt in the sensor material, an X-ray emission spectrometer instead of a solid state detector was used [4] (see Figure 2). By recording high-energy-resolution fluorescence-detected X-ray absorption spectra, the state of Pt in SnO2 based sensors could be investigated under real operating conditions.
 |
|
Figure 2. Setup of the high-energy-resolution fluorescence-detected (HERFD) XANES experiment with 5 analyser crystals at beamline ID26. The in situ cell is depicted on the top left. |
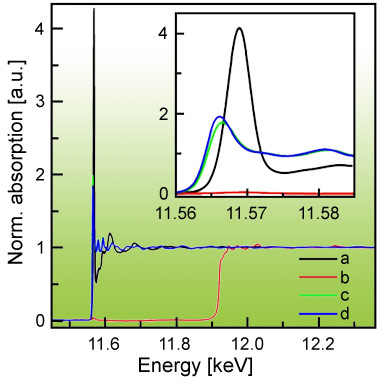
Figure 3 shows that high quality Pt XAS could be obtained without any contribution of Au. Comparing the spectra of the sensor with the ones of the Pt and PtO2 references, one clearly observes that Pt in the sensing layer is in an oxidised state. The strong white line intensity reveals that platinum is even more ionic than PtO2. Furthermore, the range-extended (i.e. beyond the Au L3 edge) EXAFS data indicate that Pt is incorporated on an atomic level in the SnO2 matrix transferring electrons to SnO2. Spectra were recorded during target gas exposure (H2 and CO) at typical sensor operation temperature (200-400°C) while simultaneously monitoring changes in the layer’s resistance.
Metallic Pt particles or clusters have been previously ascribed to the improved sensing properties when doping SnO2 with Pt. However, this study shows that all of the Pt was in its oxidised state even under rather strong reducing conditions, probably because Pt is substituting Sn in the SnO2 matrix. The enhanced selectivity and sensitivity of the SnO2 sensor upon Pt doping is thus explained by the creation of new adsorption sites on the surface and by additional electrons in the conduction band of SnO2, i.e. a bulk effect. The role played by Pt in gas sensing is therefore more complex than anticipated.
Principal publication and authors
The structure and behavior of platinum in SnO2-based sensors under working conditions, M. Hübner (a), D. Koziej (b), M. Bauer (c), N. Barsan (a), K. Kvashnina (d), M.D. Rossell (b), U. Weimar (a), and Jan-Dierk Grunwaldt (c), Angew. Chem. Int. Ed. 50, 2841–2844 (2011).
(a) Faculty of Science, Institute of Physical Chemistry, Tuebingen University (Germany)
(b) Department of Materials, ETH Zürich (Switzerland)
(c) Institute for Chemical Technology and Polymer Chemistry, Karlsruhe Institute of Technology (Germany)
(d) ESRF, Grenoble (France)
References
[1] K. Ihokura, J. Watson, CRC Press, ISBN 0-8493-2604-4 (1994).
[2] M. Schweizer, J.G. Zheng, U. Weimar, W. Göpel, N. Barsan, E. Pentia and A. Tomescu, Sensors and Actuators B 31, 71 (1996).
[3] N. Yamazoe, Sensors and Actuators B 5, 7 (1991).
[4] P. Glatzel, F. M. F. de Groot , U. Bergmann, SRN 22, 12-14 (2009).
Top image: The local structure of the platinum dopant within a working SnO2-based sensor was revealed with high-energy-resolution fluorescence-detected X-ray absorption spectroscopy and EXAFS.