- Home
- News
- Spotlight on Science
- Activation of Oxygen...
Activation of Oxygen on Gold-Alumina Catalysts: In situ High-energy Resolution
14-06-2006
Gold catalysis has received considerable attention in recent years. Particles of gold that are unsupported or supported on oxidic carriers have been reported to be very active in various oxidation reactions. Examples are the oxidation of CO to CO2, water-gas shift reaction (CO + H2O → 2CO2 + H2) and oxidation of hydrocarbons. The origin of the high catalytic activity of gold catalysts has been strongly debated and various models have been presented. The major question to be answered is how the oxygen molecule is activated.
Share
The problem was addressed at beamline ID26 by means of high energy-resolution fluorescence detected (HERFD) X-ray spectroscopy: Analysis of the near-edge structure (XANES) in conventional absorption spectroscopy of high-Z elements (ZAu=79) is limited by the lifetime broadening of the core hole excited state. However, in fluorescence detected absorption spectroscopy it is possible to record the fluorescence line with an instrumental energy bandwidth that is smaller than the core hole lifetime broadening. This results in considerably sharpened spectral features (Figure 1). A HERFD spectrum thus reveals information that is difficult or impossible to extract from a conventional absorption scan.
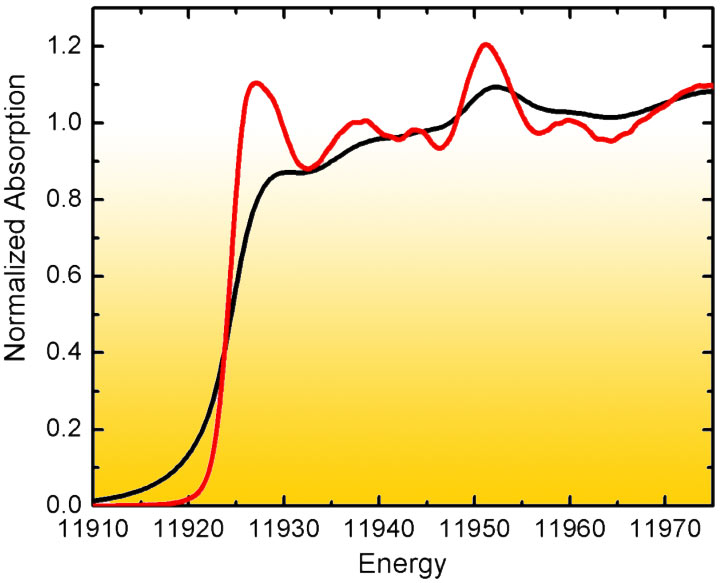 |
|
Fig. 1: Experimental L3 XANES of Au foil detected in transmission mode (black) and using high-energy resolution fluorescence detection (red). |
The study was performed at the Au L3 edge to determine the activation of oxygen on the gold catalyst. Gold nano-particles supported on a non-reducible support Al2O3 were measured under various catalytically-relevant conditions. It was found that the activation of oxygen molecules takes place on the gold nanoparticles and that CO also binds to the gold (Figure 2). The reaction dynamics were monitored by fast, conventional XANES scans with a repetition rate of 2 seconds. The rate-determining step of the catalytic reaction is thus the activation of oxygen molecules because the subsequent oxidation of CO to CO2 is very fast.
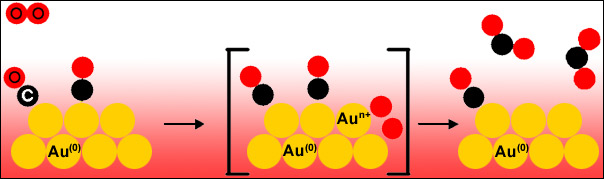 |
|
Fig. 2: Mechanism for the reaction 2CO + O2 → 2CO2 catalyzed by Au nanoparticles. |
Authors
J.A. van Bokhoven (a), C. Louis (b), J.T. Miller (c), M. Tromp (d), O.V. Safonova (e), and P. Glatzel (e).
(a) Institute for Chemical and Bioengineering, ETH Zurich (Switzerland)
(b) Laboratoire de Réactivité de Surface, UMR 7609 CNRS, Université Pierre et Marie Curie, Paris (France)
(c) BP Research Center, Naperville (USA)
(d) School of Chemistry, University of Southampton (UK)
(e) ESRF



