- Home
- News
- Spotlight on Science
- Simple alkali metal...
Simple alkali metal no longer simple
17-10-2008
Sodium is unique for its melting temperature, which is lower at very high pressures than at ambient pressure. In a recent experiment at ESRF beamline ID27, the highest-pressure single-crystal structure discovered to date has revealed that the low melting temperature of elemental sodium is associated with a number of new crystal structures. The complex phase diagram above 100 GPa of this "simple" metal suggests extraordinary liquid and solid states of sodium at extreme conditions and has implications for other "simple" metals.
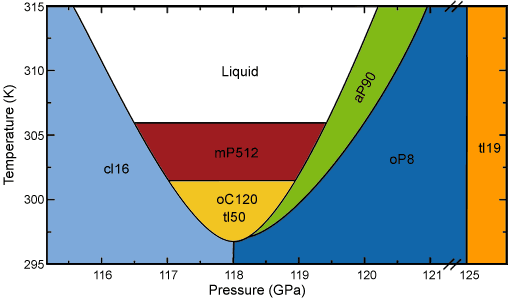
When materials are compressed, the temperature at which they melt typically increases. For example at pressures up to ~25 GPa, the melting temperatures of gases such as hydrogen and helium are the lowest and lie below 500 K [1,2]. However, the melting temperatures of both elements monotonically increase with increasing pressure, reaching ~1000 K at 100 GPa. Recently, it has been shown that although the melting temperature of sodium metal initially behaves “normally” and increases from 370 K at ambient pressure to ~1000 K at 30 GPa, it then decreases rapidly and drops to just above room temperature at pressures of 118 GPa, before increasing again [3]. The melting temperature at 118 GPa is thus lower than that at ambient pressure – a behaviour that is unique among both elements and compounds. Researchers from the University of Edinburgh carried out high-pressure single-crystal diffraction using the high intensity and micro-focused X-ray beam available at ESRF beamline ID27. They have shown that the minimum in the melting curve of sodium is associated with a large number of extremely complex crystal structures (Figure 1). Indeed, one of these structures contains more that 500 atoms in the unit cell.
 |
|
Figure 1. Observed phases of sodium in the vicinity of the minimum of the melting curve. |
The low melting temperature of sodium at 118 GPa means that it is relatively easy to melt the sample in situ and the proximity of the melting curve ensures quasi-hydrostatic conditions. It was therefore possible to grow good quality single crystals at such high pressures by slow cooling from the liquid phase. A typical single crystal diffraction image collected under these extreme conditions is presented in Figure 2. The researchers were surprised by the number of different phases found in the vicinity of the melting minimum, seven in total, and also by the way that very small changes in pressure and/or temperature resulted in transitions between the different structural forms. In addition, several of the structures were extremely complex – more so than anything else previously observed for any element, even at ambient conditions. The structures of such phases can only be determined by single-crystal methods, which the Edinburgh group have been developing as a three-year Long Term Project at the ESRF. Using these techniques, the group was able to identify the lattice parameters and the number of atoms of all seven phases and to refine the atomic positions in three of them – reaching the highest pressure ever for which the full refinement of the crystallographic structure has been carried out.
 |
|
Figure 2. Composite diffraction image showing representative data from the primitive trigonal phase with 90 atoms in unit cell. |
Early theoretical calculations on hydrogen have suggested that at the extreme compressions hydrogen might have a ground liquid state with very unusual properties and a family of the related anisotropic structures associated with its liquid state [4,5]. The results on sodium show something very similar being realised in nature giving hope that the theoretically-predicted bizarre states of hydrogen do indeed exist even though they are currently unreachable in experimental studies.
References
[1] E. Gregoryanz et al., Phys. Rev. Lett. 90, 175701 (2003).
[2] F. Datchi, P. Loubeyre, and R. LeToullec, Phys. Rev. B 61, 6535 (2000).
[3] E. Gregoryanz et al., Phys. Rev. Lett. 94, 185502 (2005).
[4] E. Brovman, Yu. Kagan, A. Kholas, Soviet Physics JETP 34, 1300 (1972).
[5] E. Brovman, Yu. Kagan, A. Kholas, Soviet Physics JETP 35, 783 (1972).
Principal publication and authors
E. Gregoryanz (a), L. Lundegaard (a), M. McMahon (a), C. Guillaume (a), R. Nelmes (a), M. Mezouar (b), Structural diversity of sodium, Science 320, 1054 (2008).
(a) Centre for Science at Extreme Conditions and School of Physics, University of Edinburgh (UK)
(b) ESRF



