- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structural biology
- Structures of PI4KIIIβ complexes show how the enzyme recruits Rab11 with its effectors
Structures of PI4KIIIβ complexes show how the enzyme recruits Rab11 with its effectors
Four mammalian enzymes phosphorylate the lipid phosphatidylinositol to produce phosphatidylinositol 4-phosphate (PI4P). One of these enzymes, PI4KIIIβ, is enlisted by a range of RNA viruses, including hepatitis C virus and enteroviruses to form PI4P-enriched viral replication organelles [1] and several compounds inhibit enteroviral replication by targeting cellular PI4KIIIβ. PI4KIIIβ is also important in the malarial life cycle, and inhibitors of the P. falciparum PI4KIIIβ are potent anti-malarial agents.
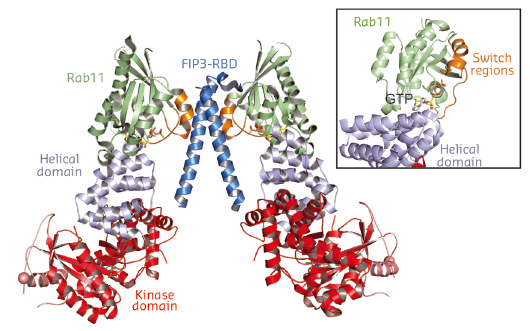
In addition to producing PI4P, PI4KIIIβ is also required for the recruitment of both the small GTPase Rab11 and its downstream effectors. To understand the structure and dynamics of PI4KIIIβ, we have employed a combination of X-ray crystallography carried out at beamlines ID23-2 and ID29 with hydrogen-deuterium exchange mass spectrometry (HDX-MS) carried out at the MRC-LMB HDX-MS facility. The HDX-MS analysis of PI4KIIIβ identified intrinsically disordered regions in the enzyme and removal of these regions enabled crystallisation of the enzyme in a complex with Rab11. The structure of the complex shows that PI4KIIIβ has a catalytic domain that resembles the phosphatidylinositol 3-kinases (PI3Ks) and that it interacts with Rab11 using a helical solenoid domain N-terminal to the kinase domain (Figure 120). These crystal contacts were confirmed in solution by HDX-MS .
 |
|
Fig. 120: The structure of PI4KIIIβ in complex with Rab11. |
PI4KIIIβ interacts with Rab11 in a contact that includes a unique direct interaction with the Rab11 GTP nucleotide, and this contact leaves the switch regions of the Rab11 largely free to interact with Rab11 effectors (see inset, Figure 120). It is the nucleotide-dependent change in conformation of these switch regions that is essential for the signalling role of Rab11. This suggests that PI4KIIIβ might be able to bind Rab11 while it, in turn, binds to its effectors. This was confirmed by a low-resolution structure of a complex of PI4KIIIβ with Rab11 and the dimeric Rab11-binding domain (RBD) of the FIP3 Rab11 effector. These structures may provide the basis for design of PI4KIIIβ inhibitors for anti-viral and anti-malarial applications.
Principal publication and authors
J.E. Burke (a,b), A.J. Inglis (a), O. Perisic (a), G.R. Masson (a), S.H. McLaughlin (a), F. Rutaganira (c), K.M. Shokat (c), R.L. Williams (a), Science 344, 1035- 1038 (2014).
(a) MRC Laboratory of Molecular Biology, Cambridge Biomedical Campus (UK)
(b) Department of Biochemistry and Microbiology, University of Victoria (Canada)
(c) Howard Hughes Medical Institute and Department of Cellular and Molecular Pharmacology, UCSF, San Francisco (USA)
References
[1] N. Altan-Bonnet and T. Balla, Trends Biochem Sci 37, 293–302 (2012).



