- Home
- Industry
- Industry news
- For better exhaust...
For better exhaust catalysts
05-06-2009
Toyota Motor Corporation (Japan and Europe) studied exhaust catalysts at the ESRF, using X-ray techniques that permitted examination of the structure of the catalyst surface and the chemical reactions taking place.
Share
What was the challenge?
To study the noble metal components of a working vehicle exhaust catalyst under in situ conditions and in real time.
What was the background to this work?
The supported metal catalyst is responsible for much of the oxidation of CO and unburned hydrocarbons in the exhaust gasses. Element specific EXAFS provides a method to examine each noble metal components individually. Current vehicle exhaust catalysts lose efficiency as they age. This is due in particular to the sintering of the supported noble metals used in the catalysts. Toyota wanted to study the sintering process in detail to improve the catalysts.
What synchrotron techniques were used?
Energy-dispersive X-ray absorption scattering was used to study the local environment and electronic structure of the Pt metallic active site while in situ transmission electron microscopy was used to study the catalyst surface. Infrared/EXAFS experiments were also used to study other major components of the catalytic system, Rh and Pd. A specialised cell was constructed to reproduce conditions within a working exhaust such as high temperatures and fluctuating oxidative and reductive gas compositions. The cell was used in conjunction with a very fast readout detector to record spectra on a millisecond timescale.
What were the results?
Experiments permitted an understanding of the sintering and redispersion of the metallic catalyst particles, and in particular of an inhibition mechanism of the platinum sintering on some metal oxide supports.
How did the synchrotron experiments help?
Analysis of the data led to the discovery of an unexpected phenomenon, i.e. an efficient oxidative redispersion of Pt nanoparticles during quick (~ 60 seconds) redox cycling. This redispersion process lead to a tangible potential for incorporation into “on board” methodology for extending vehicle catalyst lifetime through curtailing or reversing the effects of metal sintering during operation.
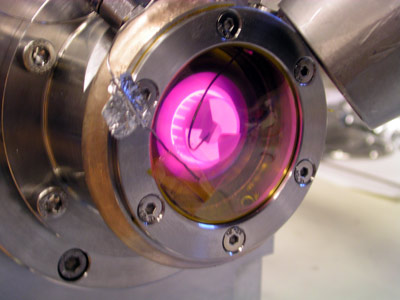 |
| Figure 1. Heating a catalyst sample in the in situ cell for time-resolved XAFS. |
Further information:
http://www.esrf.eu/UsersAndScience/Publications/Highlights/2008/XAMS/XAMS11
http://www.esrf.eu/news/spotlight/spotlight73/spotlight73/



