- Home
- Industry
- Industry news
- What are the important...
What are the important characteristics of the progesterone-receptor binding site?
10-09-2009
Schering-Plough used the ESRF MXpress service for data collection on crystals of the progesterone-receptor binding domain with bound ligands.
Share
What was the background to this work?
Mifepristone is a clinically-used antiprogestin. While it is known that mifepristone exerts its clinical effect by binding to the ligand binding domain of the progesterone receptor, there was no three-dimensional structure of it bound to the receptor. Mifepristone also binds to two other receptors within the body, which could have undesirable effects. Therefore, the aim of this research was to obtain a crystal structure of the mifepristone-progesterone receptor in order to better understand the specificity of the receptors ligand binding domain.
How did the synchrotron help?
The crystal structure was solved to 1.95 Å resolution, following data collection using the ESRF MXpress service.
What were the results?
Surprising details were found for this particular structure where mifepristone was able to bind in the conformation expected for an agonist. Prior to these studies, it was predicted that this conformation would not be possible on steric grounds, whereas only a more subtle steric conflict could be noted from the high resolution X-ray structure. These studies have extended our knowledge on the structural requirements of the progesterone receptor ligand binding domain. This will permit the design of more specific antiprogestin drugs for future clinical use.
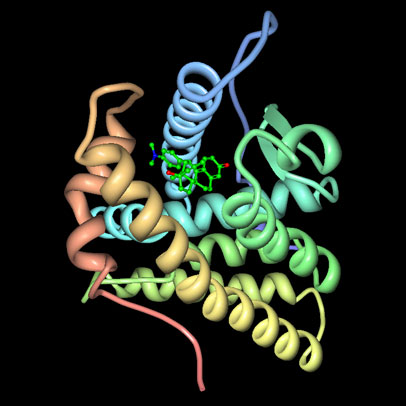 |
| Figure 1. Mifepristone (ball and stick representation) viewed within the progesterone receptor ligand binding domain (worms representation). |
For further information
The X-ray structure of RU486 bound to the progesterone receptor in a destabilized agonistic conformation, H.C.A. Raaijmakers, J.E. Versteegh, and J.C.M. Uitdehaag, J. Biol. Chem. 284, 19572 (2009).



