Fluorescence
Overview
With our microspec setup, it is possible to record fluorescence spectra of protein crystals as small as 10 μm. Various lasers in the range 266-633 nm are available as excitation source. To reduce the irradiation of the sample, which may lead to photoconversion or photodamage, fluorescence spectra are usually recorded with short laser flashes (e.g., 5 ms at 1 Hz repetition rate) using TTL signals.
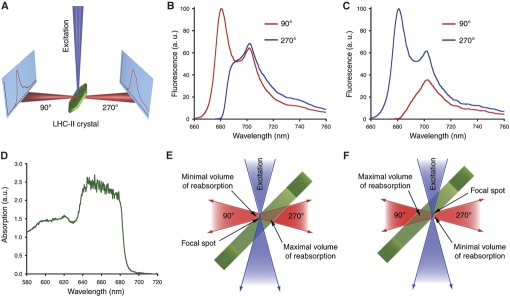
By comparing such crystal fluorescence spectra with spectra of the protein in solution (e.g., recorded on the same setup by freezing the solution in a nylon loop), it is possible to evaluate the protein's intactness in the crystalline form. If, for example, a protein's cofactor(s) are lost or altered during crystallisation, this can be reliably detected based on fluorescence spectra.
However, due to the very high optical density of protein crystals, one has to be aware of potentially misleading self-absorption effects (see example below).
Experimental setup
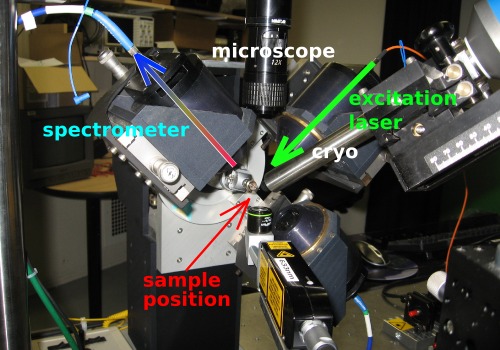
Two perpendicular objectives are used for focussing the excitation laser onto the sample and collecting the emitted light, respectively.
The setup is described in more detail in a paper by Royant et al. [J. Appl. Cryst. 40, 1105-1112 (2007)].
Software
Our spectrometers (HR2000 and HR2000+ from Ocean Optics) are used with the software SpectraSuite. A manual for SpectraSuite is available here.
Example
...under construction...
Barros et al. [EMBO J. 28, 298-306 (2009)]