- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Chemistry
- The Hydrolysis of Uranium (VI) Investigated Using EXAFS and 17O-NMR
The Hydrolysis of Uranium (VI) Investigated Using EXAFS and 17O-NMR
The hydrolysis of uranium(VI) has been the subject of extensive studies for the last 50 years because uranyl forms strong complexes with OH- in solution. A comprehensive discussion and review of the thermodynamic data is published in [1]. We investigated the structure of UO22+ as a function of pH with the aid of U LIII-edge EXAFS spectroscopy. The experiments were carried out at beamline BM20. The equipment allows excellent XAS measurements up to high k-values, 17.5 Å1 in our case.
The speciation of uranium(VI) at a total concentration of 0.05 M in slightly acidic solutions (pH 3 to 4) is dominated by the two polynuclear complexes (UO2)2(OH)22+ and (UO2)3(OH)5+. Sample A is an example from this pH region, where (UO2)3(OH)5+ is the dominant species. Structural investigations of these polymeric cations in solution at such uranium concentrations are rare. The formation of polynuclear complexes is clearly confirmed by the UU interaction at 3.81 Å (Figure 22). Approximately five oxygen atoms are coordinated in the equatorial plane at 2.41 Å.
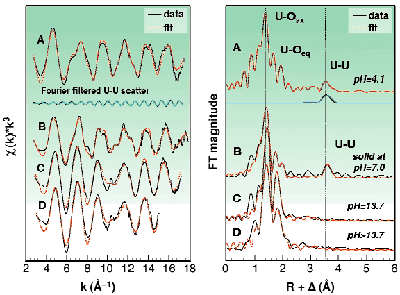 |
Fig. 22: Raw k 3-weighted EXAFS spectra for samples A to D in the binary uranyl hydroxide system including the best theoretical fits and the FT filtered UU scatter from the (UO 2) 3(OH) 5 + complex. The uranium concentration in solutions A, C, and D was 0.05 M.
|
In the pH region from 6 to 11, the U(VI) speciation is dominated by the precipitation of schoepite phases, UO2(OH)2xH2O (sample B). A longer UU distance of 3.87 Å was measured. Similar U bond lengths of around 3.9 Å were found in schoepite phases. Their structure consists of a network of UO2(OH)2 sheets, where the uranyl centres are connected via a double OH bridge.
In the alkaline pH region (sample C and D), monomeric uranium species are formed. As compared to our previous study [2], we were able to extend the k-space region from 15 Å1 in [2] up to 17.5 Å1 and thereby to increase the precision. The EXAFS measurements confirm the speciation calculations indicating that UO2(OH)42 is the major species in 0.5 M tetramethylammonium hydroxide (TMA-OH). There are two trends in the EXAFS data, the UOaxial bond length increases 1.79 Å, 1.81 Å, and 1.83 Å moving from pH 4.1 to 13.7, while the average UOequatorial bond length decreases, 2.41 Å, 2.34 Å, 2.26 Å, respectively. This indicates a stronger bonding of equatorial OH groups with increasing pH. Clark et al. [3] have presented spectroscopic evidence for the formation of a pentahydroxide complex at high TMA-OH concentrations, however with no information about the equilibrium constant. We have tested the hypothesis of Clark et al. using 17O-NMR spectroscopy with 17O-enriched "yl" oxygens. The spectrum recorded at 258 K (Figure 23) shows only one peak for UO2(OH)22 in 1 M TMA-OH. However, when increasing the hydroxide concentration to 3 M two peaks were obtained, one with the same shift as in the 1 M TMA-OH solution, 1132.2 ppm, the other at 1135.8 ppm presumably due to UO2(OH)53 (Figure 23). To conclude, the complex UO2(OH)42 has a very broad range of existence in strongly alkaline solution. At very high total concentrations of hydroxide (> 1 M TMA-OH), an additional OH ligand may coordinate in an associative reaction.
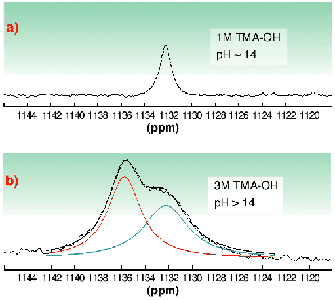 |
Fig. 23: 17O-NMR spectra of a 0.05 M UO 2 2+ solution in aqueous methanol at different TMA-OH concentrations; a) 1 M and b) 3 M (at 258 K).
|
References
[1] I. Grenthe, J. Fuger, R.J.M. Konings, R.J. Lemire, A.B. Muller, C. Nguyen-Trung and H. Wanner, in Chemical Thermodynamics of Uranium, H. Wanner, I. Forrest, (Eds.), NEA OECD, Issy-les-Moulineaux, France, p. 241 (1992).
[2] U. Wahlgren, H. Moll, I. Grenthe, B. Schimmelpfennig, L. Maron, V. Vallet and O. Gropen, J. Phys. Chem. A, 103, 8257-8264 (1999).
[3] D.L. Clark, S.D. Conradson, R.J. Donohoe, D.W. Keogh, D.E. Morris, P.D. Palmer and R.D. Rogers, C.D. Tait, Inorg. Chem., 38, 1456-1466 (1999).
Principal Publication and Authors
H. Moll (a, b), T. Reich (b) and Z. Szabó (a), Radiochim. Acta, 88, 411-415 (2000).
(a) The Royal Institute of Technology (KTH), Stockholm (Sweden)
(b) Forschungszentrum Rossendorf e.V., Dresden (Germany)



