- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Materials
- Lithium at High Pressures
Lithium at High Pressures
Lithium, the first metal in the Periodic Table, is considered a 'simple metal'. This is because at ambient conditions the motion of the conduction electrons is only weakly perturbed by interactions with the atomic cores which are arranged in a high-symmetry body centered cubic (bcc) lattice. Recently, Neaton and Ashcroft [1] predicted that dense Li at pressures below 100 GPa may undergo a sequence of structural phase transitions, leading to a low-symmetry 'paired-atom' phase (space group Cmca) with near-insulating properties. This is in sharp contrast to the intuitive expectation that application of hydrostatic pressure favors high-coordination crystal structures with metallic properties. Other experimental studies of heavy alkali metals have revealed the occurrence of high pressure phases with very unusual structures [2]. Here, the structural changes are believed to be driven by an electronic s to d transition. Experimental high-pressure investigations of lithium are of broad interest, because they are expected to uncover new aspects relevant for understanding the physics and chemical bonding of dense sp-band metals, including metallic hydrogen.
We have studied the high-pressure structural properties of Li in a diamond anvil cell using monochromatic synchrotron X-ray powder diffraction. The experiments were performed at the ID9-HP beamline. At ambient temperature and pressures above 15 GPa, Li diffuses into the diamond anvils which resulted in their repeated failure. To avoid the breaking of diamonds, the measurements were performed at low temperatures (below 200 K) in a specially designed flow cryostat.
Diffraction patterns measured at 180 K are shown in Figure 103. Li remains face centered cubic (fcc) to ~ 39 GPa (a bcc to fcc transition occurs at ~ 7.5 GPa [3]). Above 39 GPa two new phases were observed. The first, which exists only in a narrow pressure range (~2 GPa), has a trigonal structure containing one atom per unit cell (hR1 in Pearson notation). This structure can be regarded as distorted fcc and is similar to that of alpha-mercury.
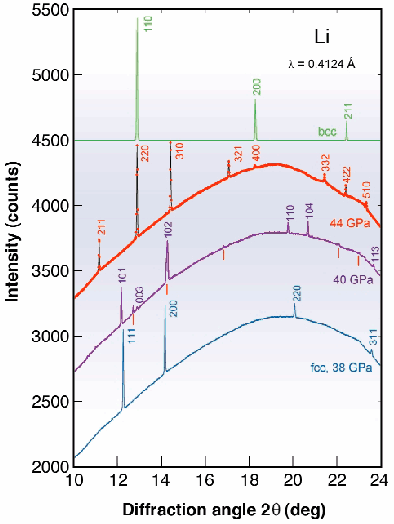 |
Fig. 103: Diffraction patterns of high-pressure phases of Li at 180 K: fcc (blue line) at 38 GPa, hR1 (pink line) with a small admixture of cI16 (red marks) at 40 GPa, cI16 measured (red dots) and calculated (black line) at 44 GPa, and hypothetical bcc pattern (green line) with a = 5.198/2 = 2.599 Å demonstrating the close relation between bcc and cI16.
|
The other new phase of lithium, however, adopts a new structure type so far not found for any other element. All diffraction lines observed can be indexed assuming a cubic unit cell (a = 5.198 Å at 44 GPa) containing 16 atoms. The only space group consistent with the systematic extinctions is I-43d. The atoms occupy the Wyckoff 16c site which has fractional coordinates (x, x, x). Rietveld refinements show that the value of x is close to 0.055 at 44 GPa, with a clear tendency to increase with increasing pressure. The new structure (cI16), which is shown in Figure 104, is stable to at least 55 GPa.
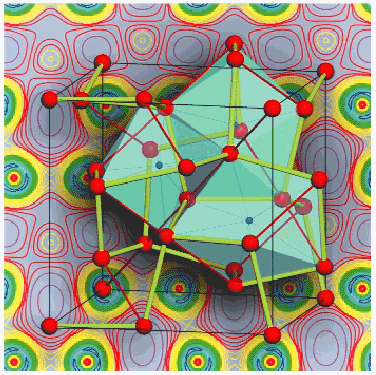 |
Fig. 104: Representation of the cI16 structure of lithium near 44 GPa. The space group is I-43d. The cubic cell contains 16 atoms occupying the Wyckoff 16c site with x ~ 0.055. Thick lines indicate the shortest interatomic distance (2.10 Å). The thin red lines connect equidistant atoms along the body diagonals. The structure can be viewed as two interpenetrating 3-connected nets. An alternative interpretation is in terms of a framework of distorted Li 8 dodecahedra. The calculated charge density distribution of Li-cI16 at a relative volume V/V 0 = 0.4 (theoretical pressure 48.8 GPa) is shown in the background. The plot is for a (100) plane and covers 4 unit cells. The color coding is blue to red/magenta for increasing electron density. The density is higher than the average value in all areas marked by red/magenta colors.
|
The crystal structure of Li-cI16 is closely related to a simple bcc lattice. The latter can be regarded as a packing of non-intersecting cylinders running parallel to the body diagonals. Moving these cylinders with respect to each other lowers the symmetry from space group Im-3m to I-43d and results in the formation of a 2 x 2 x 2 supercell of the bcc lattice. The coordination is reduced from 8 + 6 to 3 + 2 + 6. The structure resembles the cation sublattice of the binary compound Yb4As3 which is of the anti-Th3P4-type.
The structural sequence observed for lithium up to 50 GPa follows a route different from that predicted by Neaton and Ashcroft. Nevertheless, the experiments confirm their general conclusion that dense Li should become unstable towards symmetry-breaking structural rearrangements at pressures near 50 GPa.
To learn more about the stability of the cI16 structure and about its electronic properties we have performed total energy calculations using full-potential density functional theory and the generalised gradient approach. The calculated enthalpy differences fully reproduce the phase transition sequence observed experimentally (bcc - fcc - hR1 - cI16) and reveal that the cI16 structure is more stable than the 'paired-atom', Cmca phase over a large pressure range up to 165 GPa. Later calculations suggest that a phase with a Cs-IV-type structure could interfere at lower pressures. The calculated real-space valence electron density distribution for the optimised Li-cI16 structure at 48 GPa (Figure 104) is characterised by pronounced maxima in the interstitial regions, which is typical of multi-center bonding. Related to this, the calculated electronic density of states shows a deep minimum near the Fermi level, indicating semi-metallic behaviour.
References
[1] J. B. Neaton and N. W. Ashcroft, Nature, 400, 141 (1999).
[2] U. Schwarz, A. Grezechnik, K. Syassen, I. Loa, and M. Hanfland, Phys. Rev. Lett. 83, 4085 (1999); U. Schwarz, K. Takemura, M. Hanfland, and K. Syassen, Phys. Rev. Lett., 81, 2711(1998).
[3] B. Olinger and W. Shaner, Science, 219, 1071 (1983); M. Hanfland, I. Loa, K. Syassen, U. Schwarz, and K. Takemura, Solid State Commun., 112, 123 (1999).
Principal Publication and Authors
M. Hanfland (a), K. Syassen (b), N. E. Christensen (c) and D. L. Novikov (d), Nature, 408, 174 (2000).
(a) ESRF
(b) MPI für FKF, Stuttgart (Germany)
(c) Aarhus University (Danemark)
(d) A. D. Little Inc., Cambridge (USA)



