- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Surfaces and Interfaces
- Surface Study of Hardened Layers in Steels
Surface Study of Hardened Layers in Steels
Surface treatments are one of the main tools used to control the properties of different kind of materials. One important example is the case of steels where resistance to corrosion or hardness are adapted to the required standards by modifying the outer most atomic layers by processes such as nitriding [1]. In this process, nitrogen atoms are introduced into the surface layers to form metal nitrides which cause a drastic increase in surface hardness. Experimentally it is intrinsically challenging to obtain information about the specific details of this process because nitriding only causes subtle variations in chemical composition or geometric structures. Moreover, alloyed steels are rather complex systems where minor components (< 5% weight) play a fundamental role in the final mechanical properties. For this reason special surface and element-sensitive techniques are required to study them.
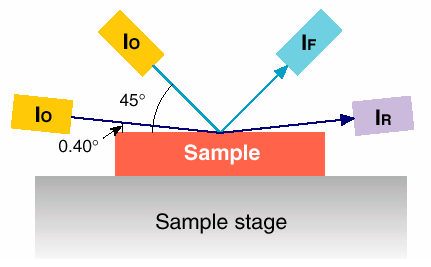
Here we present the results of an X-ray absorption spectroscopy study carried out at station BM29 on the inter-atomic scale effects of nitriding a commercially available steel. In the first component of this study, the changes induced by the nitriding treatments in the local structure of the chemically dilute alloyed elements, V, Cr, Mn, Mo, were monitored by measuring the fluorescence signal, IF, of their X-ray absorption spectrum as illustrated in the scheme showing the sample geometry.
As shown in Figure 58, it is clear that quite drastic changes in the local structure around Mo atoms were induced by the nitriding treatment, which causes the disruption of the metal phase with bcc structure, and induces the formation of nitrides, which are amongst the hardest compounds after diamond. Similar changes were recorded in the Cr, V and Mn K-edge EXAFS spectra. The sensitivity of this technique and its importance for improving our understanding of this complex system is clear when one considers a comparison with X-ray diffraction measurements. With that technique no evidence for any crystalline phases containing Mo, or any of the other alloyed elements, were found in both normal or grazing incidence investigations.
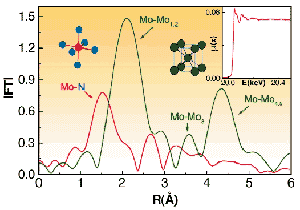 |
Fig. 58: Fourier transform of the Mo K-edge (E=20000 eV) EXAFS spectrum of a commercial steel (0.3% V; 3% Cr; 0.5% Mn; 0.8% Mo; 0.3% C; rest: Fe) before (green line) and after (red line) submitting it to a gas nitriding process. The experimental data (see inset) were recorded using the ESRF 13 element Ge solid state detector to monitor the fluorescence signal with an energy resolution better than 150 eV.
|
The fluorescence detection method utilised does not have the sensitivity to characterise depth dependency and only provides averaged structural information of the region modified by the nitriding process over a penetration depth of microns. For this reason, in a second part of the investigation, the depth dependent changes in the structure around the major elemental constituent, Fe, were then followed by measuring the REFLEXAFS spectra [2], IR, at glancing angles above and below the critical angle for which the condition of total external reflection is reached (see Figure 59). In this case, the penetration of the radiation within the solid is of the order of 30-50 Å, depending on surface roughness, and increases with glancing angles. Surface nitrided species should appear in this region. For angles of incidence above the critical angle, the penetration depth is much higher, >100 Å, and bulk phases (mainly ferrite) are dominant in this region. Our preliminary analysis of the absorption component of the reflected beam has shown that this technique can indeed provide a tool capable of detecting the extent to which nitrided phases are formed and how deep nitrogen atoms penetrate within the metal network.
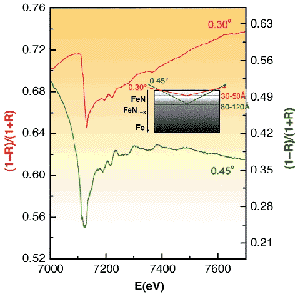 |
Fig. 59: Reflectivity around Fe K-edge (7112 eV) of the nitrided steel for two incident grazing angles: (Green line) below critical angle; (Red line) above critical angle.
|
References
[1] E.I. Meletis and S. Yan, J. Vac. Sci. Technol. A, 11, 25 (1993).
[2] P. Borthen and H.-H. Strehblow, J. Phys. Condens Matter, 7, 3779 (1995).
Principal Publication and Authors
A. Muñoz-Paez (a), J.I.F. Peruchena (a), S. Diaz-Moreno (a,b), A. Justo (a), R. Ayala (a), F. Castañeda (c), D. Bowron (b,d), S. Ansell (b) and M. Borowski (b).
(a) ICMSE-Universidad de Sevilla (Spain)
(b) ESRF
(c) Empresa Nacional Bazán, San Fernando (Spain)
(d) Now at CLRC Rutherford Appleton Laboratory (UK)



