- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Applied and Industrial Research
- Structural Transformations and Physical Properties of Zopiclone
Structural Transformations and Physical Properties of Zopiclone
Racemic zopiclone (a cyclopyrrolone hypnotic drug marketed as Zimovane® tablets) crystallises in two centrosymmetric forms: monoclinic dihydrate (I) (Figure 145) and monoclinic anhydrous (II). Zopiclone is also known to form a non-centrosymmetric orthorhombic anhydrous structure (III). Motivation for the study of the structural basis of the reversible transformation I II (and the subsequent solid-state chiral separation II
III) came from reports of significant batch-to-batch variation in physical form amongst commercial samples of zopiclone.
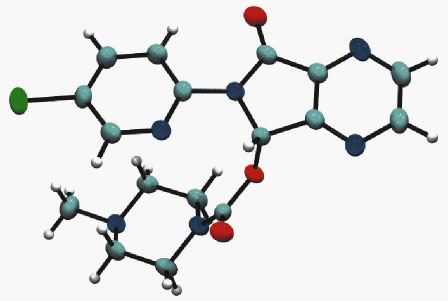 |
Fig. 145: The conformation of zopiclone in the crystal structure of form I. |
Figure 146 shows XRPD patterns collected on BM16 in the range 2.2-3.6° 2 (
= 0.8 Å) for the three forms of zopiclone. Form II was produced in situ from the starting sample of form I by breaking the sealed end of the capillary and exposing the sample to a warm N2 stream. The transformation was monitored via the growth on heating of the diagnostic (1 0 0) reflection at ca. 3.23°, which occurs at the expense of the dihydrate (1 0 0) reflection at ca. 2.96°. Further heating transformed II
III, as evidenced by the appearance of the form III (0 0 2) reflection at ca. 2.57°. The crystal structures of all three forms were determined directly from the XRPD data using the simulated annealing procedure described previously [1] which is now implemented in the DASH computer program [2].
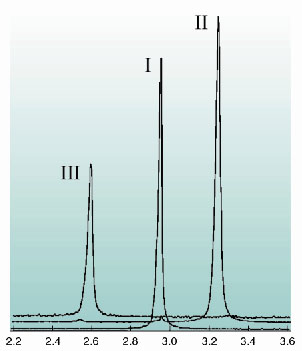 |
Fig. 146: The transformations of zopiclone on heating. |
The molecular conformations and basic packing motif in forms I and II are strikingly similar, with the zopiclone molecules arranging themselves in bilayer sheets which remain essentially unchanged in the dehydration process as the water molecules are removed from the crystal structure. The process is energetically straightforward since the principal structural change during dehydration is the close-packing of each bilayer sheet by 6.61 Å along the c axis and 1.48 Å along the a axis, both relative to the form I unit cell. This close structural similarity also accounts for the ease with which hygroscopic form II reverts to form I on exposure to high relative humidity at room temperature.
The exothermic transformation of form II III at elevated temperature is a rare example of a spontaneous resolution of enantiomers in the solid state and is accompanied by a significant change in molecular conformation. There is no doubt that the (R) and (S) enantiomers separate during the transformation, although the XRPD experiment cannot distinguish between formation of racemic twins or a racemic conglomerate either possibility is consistent with the diffraction data. However, the sharpness of the powder diffraction lines indicates domain sizes in excess of 1000 Å.
In summary, the monoclinic centrosymmetric form of zopiclone dihydrate undergoes a sequential, two-step transformation in the solid state upon heating which results in enantiomer separation. Central to detailing the transformations at molecular level was the requirement to produce a sharply diffracting sample of form II and then solve its crystal structure. In this regard the high instrumental resolution and count rate offered by BM16, plus the efficient global optimisation method, was ideally suited to the experiment and demonstrates the power of structure elucidation in 'troubleshooting' pharmaceutical processing problems.
References
[1] ESRF Highlights, 23 (1999).
[2] W.I.F. David, K. Shankland, J. Cole, S. Maginn, W.D.S. Motherwell and R. Taylor, DASH User Manual, Cambridge Crystallographic Data Centre, Cambridge (2001).
Principal Publication and Authors
N. Shankland (a,), W.I.F. David (b), K. Shankland (b), A.R. Kennedy (c), C.S. Frampton (d) and A.J. Florence (a), Chem. Commun., 2204-2205 (2001)
(a) Department of Pharmaceutical Sciences, University of Strathclyde (UK)
(b) ISIS Facility, Rutherford Appleton Laboratory (UK)
(c) Department of Pure and Applied Chemistry, University of Strathclyde (UK)
(d) Roche Discovery Welwyn, Welwyn Garden City (UK)



