- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- High-resolution X-ray Powder-diffraction Studies on Refrigerants
High-resolution X-ray Powder-diffraction Studies on Refrigerants
In a refrigeration cycle, a volatile fluid transports heat. The fluid vaporises under reduced pressure, absorbing heat that is released when the vapour recondenses under pressure. The fluid absorbs heat because the expansion during vaporisation works against the forces of attraction between the molecules, and vice-versa during condensation.
We are interested in the forces between refrigerant molecules, such as the modern hydrofluorocarbons (HFCs) and hydrofluorochlorocarbons (HCFCs), because they control the thermodynamic and heat-transporting properties of these substances. Measurements of physical properties such as viscosity, heat capacity or speed of sound can be used to derive information about the intermolecular interactions, which can then be used in theoretical simulations of the behaviour of the fluids. These interactions also determine how the molecules pack together in the solid state, but the crystal structures of these compounds have not been solved owing to the difficulties of growing single crystals at very low temperatures. A crystal structure can act as a rigorous test of the limitations and applicability of the intermolecular potentials, and moreover can be used to improve the potentials derived from physical measurements. We have grown powdered samples of the crystalline solids of a number of HFCs and HCFCs and solved their structures from high-resolution powder diffraction measurements on beamline BM16.
To carry out the measurements, we built a glass-capillary gas-condensation cell, which can be mounted on the diffractometer, cooled with a cold-gas blower, and connected to a gas-handling system (Figure 29). The gas condenses as a liquid. We seal the capillary by disconnecting the gas line, then lower the temperature to crystallise the solid. The sample is spun on the axis of the diffractometer, as is standard practice, for high-quality X-ray powder diffraction patterns measurements.
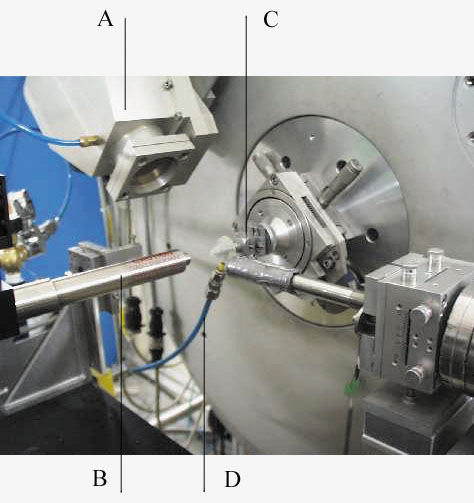 |
Fig. 29: The in situ gas-handling system built on BM16. A: partial view of the multianalyser detector arm employed at BM16. B: cold-nitrogen-gas blower, mounted coaxially with the capillary in order to have a laminar gas flow. C: goniometer head, co-axially aligned with the axis of the diffractometer, on which the sample capillary is mounted. D: the blue tube is the gas line which connects the pressurised bottle containing the sample to the capillary for the condensation. Once the capillary is filled, it is disconnected. |
As an example, the variation of the diffraction pattern of solid HFC 134a (1,1,1,2-tetrafluoroethane, CF3CH2F) is shown in Figure 30. Between the melting point (170 K) and about 110 K there are only three peaks visible in the diffraction pattern.
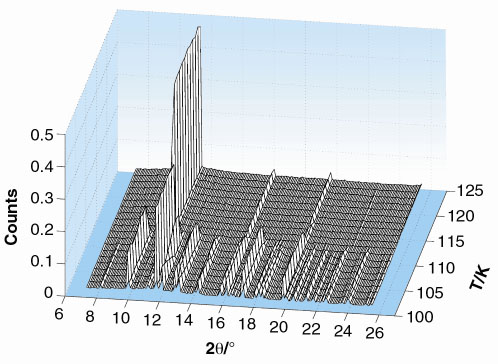 |
Fig. 30: The temperature evolution of the diffraction pattern of HFC 134a shows the order-disorder phase transition at about 110 K on cooling. |
The molecules form an arrangement where the centres of mass of the molecules are ordered, forming a cubic lattice, but their orientations are disordered, because the molecules still have sufficient thermal motion to overcome some of the forces acting between them. Below 110 K, the diffraction pattern changes abruptly. The molecules can no longer resist the forces of attraction between them; they order and change their arrangement (Figure 31) to optimise their mutual interactions. The symmetry of the crystal structure is lowered. This change of structure had not previously been reported.
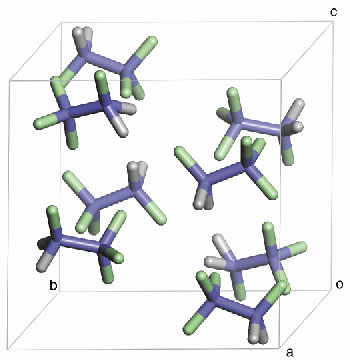 |
Fig. 31: View of the low-temperature structure of HFC 134a. |
From the study of a series of compounds, it is clear that other refrigerant molecules undergo orientational order-disorder transitions in the solid state. There is also a rich variety in the structures formed on ordering below the transitions, as molecules align under the influence of dipolar and higher-order interactions, and the van-der-Waals forces between them. To model this behaviour from intermolecular potentials, both of the ordered structures and the transitions to the orientationally-disordered form, represents a stiff challenge to the techniques of theoretical chemistry and molecular dynamics simulation.
Principal Publication and Authors
M. Brunelli and A.N. Fitch, J. Appl. Cryst., submitted.
ESRF



