- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- Sulphur at High Pressures and Temperatures
Sulphur at High Pressures and Temperatures
Sulphur has the largest number of allotropes of any element, forming n-member-ring and helical structures. Of the helical structures, few complete solutions exist. Vezzoli and Walsh [1] have proposed that their phase XII (assigned as a fibrous form) is present at pressures above ~ 3 GPa and temperatures above 400°C. We undertook an experiment using the Paris-Edinburgh press on ID30 to try to elucidate the structure of this high-p,T form and its relationship to the reported Fddd structure, made of 8-member rings, which is the stable structure under ambient conditions.
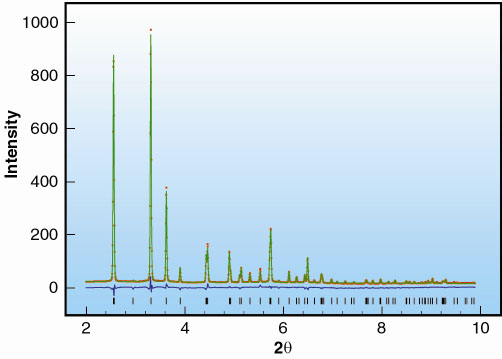 |
Fig. 32: In situ pattern obtained at 3 GPa and 400°C, red, calculated pattern, green, and difference, blue, obtained for our helical model. Peak positions are indicated by vertical markers. |
We found that the Fddd phase transforms to produce a simpler diffraction pattern (Figure 32), indicative of an increase in symmetry and a smaller unit cell, which could be indexed as hexagonal, indeed altogether different from the previous description of phase XII. We solved the structure from the powder-diffraction data, measured in situ, using a simulated annealing and global optimisation algorithm, which resulted in non-chiral helical chains of sulphur in the trigonal space group P3221. There are two unique sulphur sites (6c and 3b) per unit cell, Figure 33. As expected (see detailed discussion on proposed structures of helical forms of sulphur in Prins et al. [2]), the structure is very similar to that of trigonal selenium, having a three-atom chain repeat along the c-axis; but differs in that selenium has only one Se site per unit cell [3]. The relationship between this new phase and that described as phase XII became clear when we slowly reduced the pressure on the sample after temperature quenching. A back transformation occurs to a metastable 4.04 Å fibrous-like phase at pressures less than ~ 0.5 GPa (which, over a 9 month period, shows signs of sharpening very slowly into a pattern with features akin to the Fddd phase).
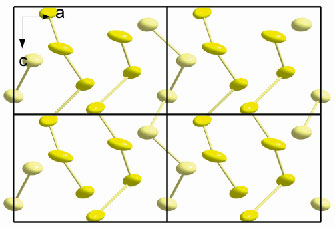 |
Fig. 33: Unit cell contents, with c-axis vertical, of the high-p, T form of sulphur showing two distinct helices; the central one is composed entirely of S2, light yellow, on the 3b site around the vertex of the cell and the other, S1 dark yellow, on the 6c site rotating around the 32-screw axis. |
We can only conclude that the effects of annealing and polymorphism of quenched phases has added complexity to the phase diagram of S, (e.g. see [1]), whereas our more recent in situ work on ID30 has indicated that it is really much more simple. We hope that this study serves to highlight the necessity of in situ observation in p, T phase diagram construction and the ability to solve structures by powder diffraction methode with clean data collected using the Paris-Edinburgh/multi-slit collimator assembly at non-ambient condutions.
References
[1] G.C. Vezzoli and P.J. Walsh, High Temp. High Press., 9, 345-359 (1977).
[2] J.A. Prins, J. Schenk and L.H.J. Wachters, Physica, 23, 746-752 (1956).
[3] P. Cherin and P. Unger, Inorg. Chem., 6, 1589-1591 (1967).
Principal Publication and Authors
W.A. Crichton, G.B.M. Vaughan and M. Mezouar, Z. Krist., 216, 417-419 (2001).
ESRF



