- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- Structure of Neptunium(VII) Complexes at High pH
Structure of Neptunium(VII) Complexes at High pH
Information on the structure of complexes in solution is essential for the understanding of chemical equilibria (complex formation and redox reactions) and chemical reactivity. In this study we have used a combination of experimental EXAFS data from BM20, the Rossendorf Beamline at the ESRF, and quantum chemical methods to obtain information on the structure of Np(VII) complexes formed in aqueous solution at high pH. The results provide a clear indication that the structure is an octahedral oxo-complex with the composition NpO4(OH)23-. This information, together with structural information about the Np(VI) complex NpO2(OH)42- formed in the same pH range, has also made it possible to explain the reversibility of the Np(VII)/Np(VI) redox couple.
There are two prior EXAFS studies of the structure of the Np(VII) complex formed in alkaline solution. Clark et al. [1] suggested the composition NpO2(OH)4- with square bipyramidal geometry, whilst Soderholm et al. [2] suggested NpO4(OH)23-. Both these experiments were made using fluorescence detection over a limited k-space range (< 12 Å1), resulting in a fairly large uncertainty in the interpretation. In order to make a choice between the possible structures, we made a new set of EXAFS experiments with a 0.015 M Np(VII) solution up to k = 17 Å1 in transmission mode that provides more precise data. The XANES spectrum was also recorded and differed significantly from that of Np(V), and Np(VI), indicating that there is no linear NpO2 unit present like in Np(V) and Np(VI). The EXAFS data and the structure of NpO4(OH)23-, the complex identified by this study, are given in Figure 45 and Figure 46, where the latter has been obtained using quantum chemical methods as described below.
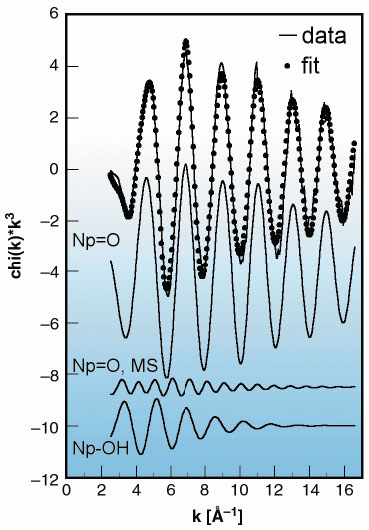 |
Fig. 45: (Top) Np LIII -edge k3-weighted EXAFS data (continuous lines) including the best fit (dotted lines) for 0.015 M Np(VII) in 2.5 M NaOH; (Below) Deconvoluted oscillations from single scattering on Np=O and Np-OH as well as from the MS path. |
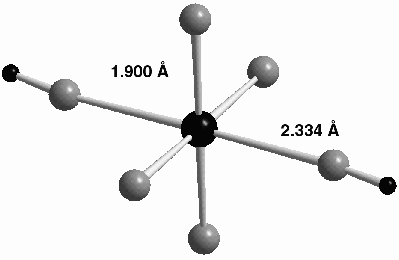 |
Fig. 46: The optimised geometry of NpO4(OH)23-, where the black central atom denotes Np(VII) , the grey atoms oxygen and the small black atoms hydrogen. |
The detailed structural model was obtained using quantum chemical methods (Hartree-Fock and DFT with energy-consistent relativistic effective core potentials). The structure was determined both in the gas-phase and by using a continuum model (CPCM) for the solvent. The theoretical results are in excellent agreement with the EXAFS experiments; the difference in the Np(VII)O bond distances is less than 0.01 Å. In addition the quantum chemical model provides a detailed three-dimensional structure.
The formal potential of the Np(VII)/Np(VI) redox couple varies with the hydroxide ion concentration, and this observation also indicates that there are two OH groups more in the Np(VI) complex than in the Np(VII) species. Together with the known structure of the corresponding U(VI) complex in strongly alkaline solution, UO2(OH)42-, this observation supports further the conclusion that the Np(VII) and Np(VI) complexes have the stoichiometry NpO4(OH)23- and NpO2(OH)42-, respectively.
The solution structures of NpO4(OH)23- and NpO2(OH)4- are both octahedral with fairly small differences in the NpO and the NpOH distances between Np(VII) and the Np(VI). This is 0.07 Å for NpO and 0.08 Å for NpOH. NpO2(OH)4- can be looked upon as the protonated form of NpO4(OH)23-. The fast proton transfer reaction and the small changes in bond length are prerequisites for fast electron transfer between Np(VII) and Np(VI) at an inert electrode and thereby to a reversible redox potential.
References
[1] D.L. Clark, S.D. Conradson, M.P. Neu, P.D. Palmer, W. Runde and C.D. Tait, J. Am. Chem. Soc. 119, 5259 (1997).
[2] L. Soderholm, M.R. Antonio, C.W. Williams, J.C. Sullivan, S.R. Wasserman and J.-P. Blaudeau, J. Am. Chem. Soc. 123, 4346 (2001).
Principal Publication and Authors
H. Bolvin (a), U. Wahlgren (b), H. Moll (c), T. Reich (c), G. Geipel (c), T. Fanghänel (c) and I. Grenthe (d), J. Chem. Phys. A, 105, 11441-11445 (2001).
(a) Institute of Chemistry, University of Tromsø (Norway), on leave from IRSAMC, Université Paul Sabatier, Toulouse (France).
(b) Institute of Physics, Stockholm University (Sweden)
(c) Institute of Radiochemistry, Forschungszentrum Rossendorf e.V., Dresden (Germany)
(d) Inorganic Chemistry, Department of Chemistry, Royal Institute of Technology (KTH), Stockholm (Sweden)



