- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2003
- Macromolecular Crystallography
- Human Angiotensin I Converting Enzyme Structure Revealed
Human Angiotensin I Converting Enzyme Structure Revealed
Since the 1980s, inhibitors of angiotensin converting enzyme (ACE inhibitors) have achieved great success as first-line therapy for cardiovascular diseases, including high blood pressure, heart failure, coronary artery disease, and kidney failure. These anti-hypertensive drugs were not designed based on any knowledge of the three-dimensional structure of ACE, but on an assumed mechanistic homology to carboxypeptidase A, whose structure has been known for some time. However, prolonged administration of current ACE inhibitors leads to several undesirable side effects, such as a persistent dry cough, headaches and dizziness [1].
There are two isoforms of ACE in the human body, somatic and testicular. Somatic ACE exists in most cells in the body and testicular ACE, which is half the size of somatic ACE, is found only in the testes. Both convert inactive angiotensin I to its active form, angiotensin II, which stimulates blood vessel constriction. Both forms of ACE also inactivate bradykinin, which stimulates blood vessel dilation. The X-ray structure of testicular ACE, and its complex with the widely used ACE inhibitor lisinopril, at 2.0 Å resolution has been elucidated. This structure was determined using the anomalous scattering of the bound Zn atom at beamline BM14 of the ESRF.
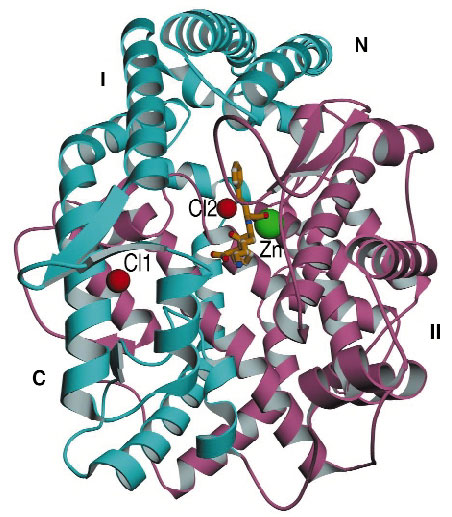
The three-dimensional structure reveales that ACE is composed of a-helices for the most part, and incorporates a zinc ion and two chloride ions (Figure 33). In fact it bears little resemblance to carboxypeptidase A except in the active site zinc-binding motif. Instead, it resembles rat neurolysin and Pyrococcus furiosus carboxypeptidase, despite sharing little amino-acid sequence similarity with these two proteins. This similarity extends to the active site, which consists of a deep, narrow channel that divides the molecule into two subdomains. On top of the molecule is an amino-terminal 'lid', which seems to allow only small peptide substrates (2530 amino acids) access to the active site cleft this accounts for the inability of ACE to hydrolyse large, folded substrates.
 |
|
Fig. 33: Crystal structure of human testicular ACE with the inhibitor (lisinopril) molecule bound at the centre of the molecule. The green sphere represents the zinc ion and the red spheres represent the bound chloride ions. |
Somatic ACE consists of two parts (called the N- and C-domains), each having a different function, and current drugs inhibit both domains. The newly described structure of testicular ACE (which is identical to the C-domain of somatic ACE) will now serve as a 'template' for the design of next-generation, domain-selective ACE inhibitors with the potential for greater efficacy, fewer side effects and new treatment indications for hypertension.
References:
[1] K.R. Acharya, E.D. Sturrock, J.F. Riordan and M.R.W. Ehlers, Nature Reviews- Drug Discovery. 2, 891 (2003).
Principal Publication and Authors:
R. Natesh (a), S.L.U. Schwager (b), E.D. Sturrock (b) and K.R. Acharya (a), Nature 421, 551 (2003).
(a) University of Bath, Bath (UK)
(b) University of Cape Town (South Africa)



