- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Soft Condensed Matter
- Nucleation and Growth Kinetics of Brome Mosaic Virus Microcrystals
Nucleation and Growth Kinetics of Brome Mosaic Virus Microcrystals
Understanding the interaction potentials that govern crystal formation is an essential step for X-ray structure determination. Brome Mosaic Virus (BMV) and polyethylene glycol (PEG) mixtures were chosen as a crystallisation model since their phase diagram presents a solid precipitated phase at high polymer concentration, known to be made of the synchronous formation of a large number of microcrystals[1]. Indeed, the addition of neutral polymers like PEG to biomacromolecular solutions results in an attractive potential (the depletion attraction) dependent upon polymer size and concentration. When the attraction increases, a fluid-fluid or fluid-solid phase separation eventually occurs.
The onset of BMV crystal nucleation and growth was observed for the first time using time-resolved X-ray scattering by the synchronisation of a fast stopped-flow mixing system (Bio-Logic) and the fast detector of ID02, for virus concentrations from 20 to 2.5 mg/ml and PEG concentrations down to 5% w/v. Due to the differences in molecular weights and contrasts of BMV and PEG, only the virus scattering was observed, in solution, in crystals and, possibly, in intermediary states. Twenty measurements of 50 ms each, exponentially spaced in time, were adequate to cover the process and avoid radiation damage.
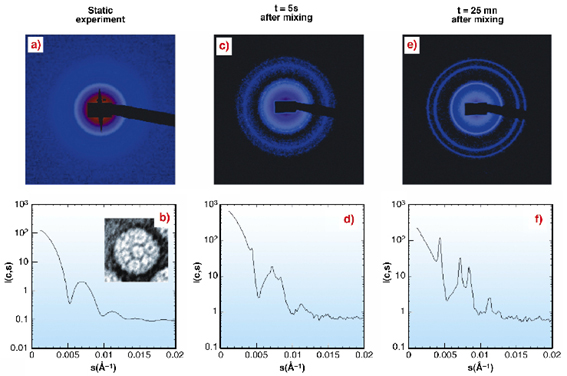
Individual spots, i.e. Bragg peaks, arising from individual microcrystals appeared about one second after mixing (Figures 55c-d). After 20-60 seconds, the increasing number of spots built the diffraction rings of a crystal powder diagram as seen on the Figures 55e-f. The intensity of the rings grew for about ten minutes until there was almost no virus left in the solution. Interference rings, which would be a signature of an amorphous intermediate state, were not detected. This indicaties that only two phases were present during the time-resolved experiment: soluble viruses and microcrystals.
 |
|
Fig. 55: Scattering intensities of Brome Mosaic Virus (top: 2D images; bottom: corresponding azimuthally regrouped and normalised scattered intensities); a,b: form factor obtained without PEG (20 frames at 2.5mg/ml). b-insert: electron micrograph of a negative staining BMV particle. The BMV is a T3 icosahedral RNA plant virus of 268 Å in diameter and a molecular weight of 4.6x106 Da. c-f: nucleation and growth of BMV crystals shows the appearance of individual microcrystal spots and Bragg peaks (BMV 10mg/ml and 5% PEG 20000). |
Dividing the intensity curves by the form factor to remove the contribution of the virus shape emphasised the structure factor (Figure 56a) and confirmed that only the crystallised form of the BMV was present.
 |
|
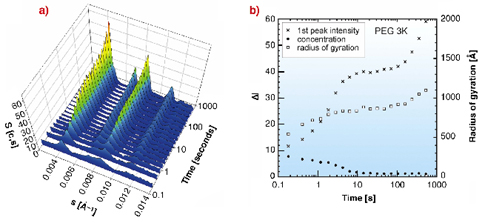
Fig. 56: BMV 20 mg/ml and 10% PEG 3K evolution of a) structure factors and b) peak intensity, BMV concentration decrease (measured from the plateau value, from 0 to 0.0038 Å-1) and microcrystal average radius of gyration (from the peak shape). Nucleation is achieved between 12 to 100 seconds after the mixing. The initial value measures the BMV concentration at the beginning of the experiment, while the final plateau value is close to the virus solubility. For each PEG the crystalline system was found to be face centered cubic (unit cell side close to 391 Å). |
The time evolution of the three parameters of Figure 56b was used to obtain the kinetic parameters of microcrystal nucleation and further growth. Both events induce a decrease in the concentration of soluble virus in solution and contribute to the increase of the peak intensities. The time lag necessary for Bragg peaks to become visible defines the onset of nucleation and varies from one to a few seconds according to the supersaturation, i.e. the BMV concentration. Then the diffraction peaks went on growing in essentially two steps corresponding to nucleation and crystal growth. A higher PEG concentration was able to counterbalance a lower molecular range to produce crystals.
The size of the critical nuclei was evaluated with the PEG 3K conditions. Il should be equal to or smaller than the smallest microcrystal (radius of gyration of about 500 Å). Taking 276 Å as the virus diameter and assuming that 70 % of the nucleus volume is occupied by the virus in the fcc system, the number of viruses in the critical nucleus is therefore calculated to be ![]() 36.
36.
These novel experiments were designed to address the question of whether the initial formation of dense liquid droplets could precede or favour nucleation. Our results demonstrate that in the case of BMV the formation of periodic order may take place without any long life concentrated "liquid-like" droplets.
References
[1] M. Casselyn, J. Perez, A. Tardieu, P. Vachette, J. Witz, H. Delacroix, Acta Crystallogr. D57:1799-1812 (2001).
Principal Publication and Authors
M. Casselyn (a), A. Tardieu (b), H. Delacroix (a) and S. Finet (c), Biophys. J. 87, 2737 (2004).
(a) BioInformatique Structurale - Centre de Génétique Moléculaire, Gif-sur-Yvette (France)
(b) Laboratoire de Minéralogie et Cristallographie, Paris (France)
(c) ESRF



