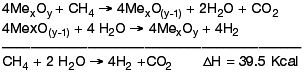- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Materials Science
- In situ Study of Methane Anaerobic Combustion on Iron Oxides: Towards a Clean Hydrogen Production Process
In situ Study of Methane Anaerobic Combustion on Iron Oxides: Towards a Clean Hydrogen Production Process
Hydrogen will be one of the most important fuels in a future sustainable economy. In the medium term, hydrogen production will still rely on fossil fuels, whose environmental impact has to be lowered both by modifying actual technologies and/or developing new ones capable of mitigating CO2 emissions. ENI is developing a clean process for H2 production from fossil fuels based on the properties of selected oxides that, once reduced with hydrocarbons, are capable of being re-oxidised by splitting H2O into H2 and [O], which in turn re-oxidises the solid closing the loop [1].
|
|
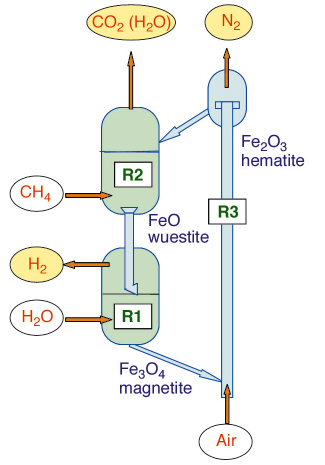
A redox solid circulating among three fluidised bed reactors is the asset developed to exploit the reaction scheme (Figure 52).
 |
|
Fig. 52: Conceptual reactors design to exploit the redox concept on iron oxide based circulating solid. A concentrated stream of CO2/H2O (ready to be buried) is produced in reactor 2, when the solid is reduced by methane (Fe2O3/FeO). A stream of H2 (virtually carbon free) is produced in reactor 1, when the solid is partly oxidised with water (FeO/Fe3O4). Thermal balance is achieved in reactor 3, where the solid is fully reoxidised (Fe3O4/Fe2O3). |
We carried out a combined X-ray diffraction (XRD) / mass spectrometry (MS) experiment at the Italian CRG BM08 “GILDA” beamline, to study the structural evolution of an iron based solid exposed to methane under anaerobic combustion conditions as a first step of such a redox cycle.
The CeO2/Fe2O3 solid investigated was exposed to a high temperature (~ 1000 K) CH4 reducing flux followed by a re-oxidation in air. The sample was contained in an open quartz capillary. One side was connected to a gas injecting system (CH4/Ar mixture, air and Ar for purging cycles), the other to a mass spectrometer (MS). X-ray powder diffraction was collected by a translating image plate system with a time resolution of one minute.
 |
|
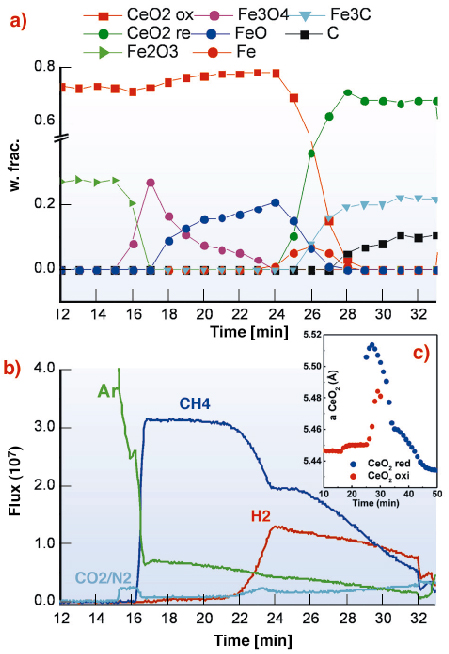
Fig 53: Evolution during the reduction cycle: a) Quantitative phase analysis; b) Evolved gases; c) Plot of the CeO2 cell parameter. |
All the collected powder patterns were analysed by the Rietveld method, obtaining a complete structure characterisation of the process in terms of quantitative analysis of the crystal phases involved (see Figure 53a). As soon as the CH4 enters the capillary, a series of redox reactions take place. Iron present as Fe3 + in Fe2O3 is progressively reduced to Fe+2Fe+32O4 (magnetite), Fe+2O (wuestite) and finally to Fe. The appearance of metallic iron in the XRD signal coincides with the H2 emission and the decrease of the flow of outgoing CH4 (Figure 53b) detected by MS. This is direct evidence of methane cracking (CH4 ![]() C + 2H2) catalysed by iron. The carbon produced by this reaction appears two minutes later as Fe3C, which replaces iron in catalysing the cracking, and as a polymorph of graphite. We have clear evidence that CeO2 participate to the reduction process. It is known that in a reducing environment ceria may convert into a non-stoichiometric reduced form (CeO2-x), whose crystal structure has the same symmetry but a larger unit cell. By mapping the variation of CeO2 unit cell parameter, a small jump corresponding to CH4 injection and a large jump corresponding to H2 emission can be detected. The latter change is simultaneous with a splitting of the ceria diffraction peaks that can be interpreted as a mixture of two reduced ceria phases with different defectivity (Figure 53c). The stoichiometry of the reduced ceria is proportional to the unit cell variation and seems to be temperature independent [2]. The estimated compositions are CeO1.99 after the CH4 injection and CeO1.88 for the most reduced form appearing after H2 emission. Peak shape analysis demonstrates that during CH4 combustion CeO2 undergoes re-crystallisation since the full width half maxima of CeO2 peaks constantly decrease. The oxidation follows the reverse path of the reduction except for the direct oxidation of Fe3 C to FeO without the intermediate formation of iron. The final solid has the same composition as the starting mixture demonstrating that the cycle is totally reversible.
C + 2H2) catalysed by iron. The carbon produced by this reaction appears two minutes later as Fe3C, which replaces iron in catalysing the cracking, and as a polymorph of graphite. We have clear evidence that CeO2 participate to the reduction process. It is known that in a reducing environment ceria may convert into a non-stoichiometric reduced form (CeO2-x), whose crystal structure has the same symmetry but a larger unit cell. By mapping the variation of CeO2 unit cell parameter, a small jump corresponding to CH4 injection and a large jump corresponding to H2 emission can be detected. The latter change is simultaneous with a splitting of the ceria diffraction peaks that can be interpreted as a mixture of two reduced ceria phases with different defectivity (Figure 53c). The stoichiometry of the reduced ceria is proportional to the unit cell variation and seems to be temperature independent [2]. The estimated compositions are CeO1.99 after the CH4 injection and CeO1.88 for the most reduced form appearing after H2 emission. Peak shape analysis demonstrates that during CH4 combustion CeO2 undergoes re-crystallisation since the full width half maxima of CeO2 peaks constantly decrease. The oxidation follows the reverse path of the reduction except for the direct oxidation of Fe3 C to FeO without the intermediate formation of iron. The final solid has the same composition as the starting mixture demonstrating that the cycle is totally reversible.
References
[1] U. Cornaro, D. Sanfilippo US2004152790-A1.
[2] H. Chiang, R.N. Blumenthal, R.A. Fournelle, Solid State Ionics 66, 85-95 (1993).
Principal Publication and Authors
M. Gemmi (a), M. Merlini (a), U. Cornaro (b), D. Ghisletti (b), G. Artioli (a) J. Appl. Cryst (2005) 38, 353-360.
(a) Dipartimento di Scienze della Terra “A. Desio”, Università degli Studi di Milano (Italy)
(b) Eni Tecnologie Spa, San Donato Milanese (Italy)