- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structural biology
- Molecular basis for activation of Rho GTPases by DOCK guanine nucleotide exchange factors
Molecular basis for activation of Rho GTPases by DOCK guanine nucleotide exchange factors
Malignant tumour cells are characterised by their abilities to invade local tissues and migrate (metastasis) to sites separate from the primary tumour. Metastasis is the main factor accounting for cancer treatment failure and is responsible for 90% of cancer deaths. Thus, intensive research efforts are directed at understanding the cellular and molecular basis of tumour cell motility and invasion.
Eukaryotic cells undergo cell migration through dynamic changes in their actin-based cytoskeleton, in a process controlled by small GTPases of the Rho family and coupled to reversible protein phosphorylation. Rho GTPases inter-convert between inactive GDP-bound and active GTP-bound states, a process controlled by guanine nucleotide exchange factors (GEFs) that catalyse exchange of GDP for GTP at the nucleotide-binding site. Two distinct families of Rho GEFs, Dbl homology (DH) and DOCK, activate Rho GTPases [1,2]. DOCK proteins have been implicated in the activation of Rac and Cdc42 in cell migration, morphogenesis and phagocytosis [3,4] and have been shown to be important components in tumour cell movement and invasion. DOCK9 belongs to the D subfamily of Rho GEFs that specifically activate Cdc42.
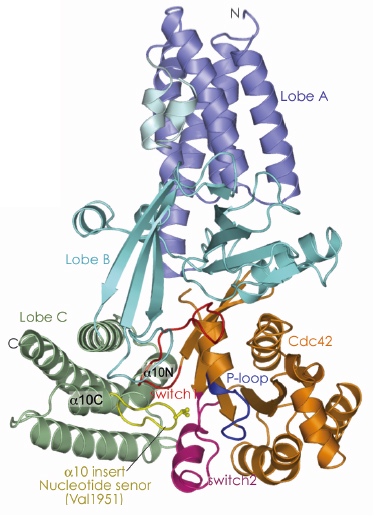
Prior to our work, the structural basis of nucleotide release catalysed by the DH family of Rho GEFs had been established but the molecular mechanisms of DOCK proteins were unknown. Moreover, the structural events that occur when GTP binds to a GEF-GTPase complex, immediately preceding discharge of the activated GTPase, had not been defined. To understand these processes we investigated the catalytic GEF domain of DOCK9. The catalytic domains of GEFs form stable complexes with nucleotide free GTPases. To determine the structure of a DOCK9 DHR2 domain (DOCK9DHR2) in complex with the Rho GTPase Cdc42, we collected single wavelength anomalous dispersion data at beamline ID29. We found that the DOCK9 DHR2 domain, which has a different structure from DH GEF catalytic domains, is organised into three lobes of roughly equal size (lobes A, B and C), with the Cdc42-binding site and catalytic centre generated entirely from lobes B and C (Figure 106). Lobe A is an anti-parallel array of five ![]() -helices and forms extensive contacts with lobe B to stabilise the DOCK9 DHR2 domain. Lobe B adopts an unusual architecture comprising two anti-parallel ß-sheets disposed in a loosely packed orthogonal arrangement. Lobe C is a four-helix bundle. Helix a10 of lobe C, the most conserved region of DHR2 domains is interrupted by a seven-residue loop - the
-helices and forms extensive contacts with lobe B to stabilise the DOCK9 DHR2 domain. Lobe B adopts an unusual architecture comprising two anti-parallel ß-sheets disposed in a loosely packed orthogonal arrangement. Lobe C is a four-helix bundle. Helix a10 of lobe C, the most conserved region of DHR2 domains is interrupted by a seven-residue loop - the ![]() 10 insert (nucleotide sensor).
10 insert (nucleotide sensor).
 |
|
Fig. 106: View of the crystal structure of the nucleotide-free DOCK9DHR2-Cdc42 complex. Lobes A, B, and C are coloured in blue, cyan and green respectively. The |
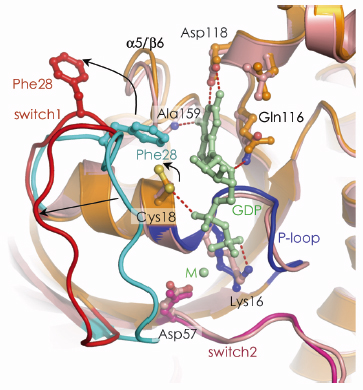
The first step of the exchange cycle is to promote nucleotide release by inducing a reduced affinity for nucleotide (Figure 107). This is achieved as follows: Upon binding to DOCK9DHR2, first the conformational transition of switch 1 of Cdc42 exposes the nucleotide-binding site. Second, this movement of switch 1 is linked to rotation of the P-loop Cys 18 thiol group that disrupts a hydrogen bond with the nucleotide ![]() -phosphate. Third, by intruding into the GTPase nucleotide-binding site, Val 1951 of the DOCK9DHR2 a10 insert directly occludes nucleotide-coordinated Mg2+. Because magnesium enhances nucleotide affinity by neutralising the negatively charged phosphate groups, its exclusion profoundly reduces nucleotide affinity.
-phosphate. Third, by intruding into the GTPase nucleotide-binding site, Val 1951 of the DOCK9DHR2 a10 insert directly occludes nucleotide-coordinated Mg2+. Because magnesium enhances nucleotide affinity by neutralising the negatively charged phosphate groups, its exclusion profoundly reduces nucleotide affinity.
 |
|
Fig. 107: The mechanism of DOCK9DHR2-mediated nucleotide release. The conformational transition of switch 1 of Cdc42 is shown with an arrow indicating the motion of the Cys18 thiol. |
To understand the second step of the exchange cycle, loading of GTP-Mg2+ and discharge of the activated Cdc42-GTP-Mg complex, we determined the structure of a GTP-bound ternary complex (data collected at DLS 104). We found that in this structure, Mg2+ bound to GTP caused a displacement of the ![]() 10 insert that removes the clamp from switch 1, and induces a set of interdependent conformational changes within other regions of the DOCK9DHR2-Cdc42 interface. This reorganisation of the complex disrupts DOCK9DHR2 contacts to switch 1. In this process activation of Cdc42 is detected by the presence of Mg2+ tightly bound to GTP, triggering displacement of the
10 insert that removes the clamp from switch 1, and induces a set of interdependent conformational changes within other regions of the DOCK9DHR2-Cdc42 interface. This reorganisation of the complex disrupts DOCK9DHR2 contacts to switch 1. In this process activation of Cdc42 is detected by the presence of Mg2+ tightly bound to GTP, triggering displacement of the ![]() 10 insert and propagation of conformational changes to the DOCK9DHR2-Cdc42 interface. Because GDP does not induce equivalent conformational changes, GTP may specifically promote discharge of the GTP-bound Cdc42 from DOCK9DHR2 to complete the catalytic exchange reaction. Thus, the
10 insert and propagation of conformational changes to the DOCK9DHR2-Cdc42 interface. Because GDP does not induce equivalent conformational changes, GTP may specifically promote discharge of the GTP-bound Cdc42 from DOCK9DHR2 to complete the catalytic exchange reaction. Thus, the ![]() 10 insert of DOCK proteins acts as sensor of the GDP and GTP bound states of Rho GTPases to mediate the catalytic cycle of GDP release and subsequent discharge of the GTP-bound, activated GTPase.
10 insert of DOCK proteins acts as sensor of the GDP and GTP bound states of Rho GTPases to mediate the catalytic cycle of GDP release and subsequent discharge of the GTP-bound, activated GTPase.
References
[1] J.L. Bos, H. Rehmann and A. Wittinghofer, Cell 129, 865-877 (2007).
[2] K.L. Rossman, C.J. Der and J. Sondek, Nat Rev Mol Cell Biol 6, 167-180 (2005).
[3] J.F. Cote and K. Vuori, Trends Cell Biol 17, 383-393 (2007).
[4] N. Meller, S. Merlot and C. Guda, J Cell Sci 118, 4937-4946 (2005).
Principal publication and authors
J. Yang, Z. Zhang, S.M. Roe, C.J. Marshall and D. Barford, Science 325, 1398-1402 (2009).
Institute of Cancer Research, Chester Beatty Laboratories, London (UK)



