- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Electronic structure and magnetism
- Emerging magnetic moment in Pt13 nanoparticles
Emerging magnetic moment in Pt13 nanoparticles
A fascinating property of matter at the nanosize scale is the appearance of new physical phenomena which do not exist in the bulk. For example, bulk Pt has a very weak magnetic response, only present if a magnetic field is applied since it is a Pauli paramagnet. It is known that the presence of a magnetic material such as cobalt may induce a magnetic moment on Pt, this has been evidenced by X-ray magnetic circular dichroism (XMCD) of Pt covering a Co nanoparticle [1]. However, it has recently been found that small Pt13 clusters may develop a magnetic moment when embedded in NaY zeolite [2]. A number of questions remained open after this exciting report, based on conventional magnetisation measurements: what is the contribution to the spontaneous moment coming from the orbital moment, and whether the observed magnetic moment could have arisen from a spurious source such as Fe contamination of the zeolite.
In this work we show without any ambiguity that Pt13 nanoparticles do indeed have a magnetic moment at low temperature, by means of XMCD at the Pt L2,3 edges. The sample, which consisted of NaY zeolite charged with 6% by weight of Pt, was air sensitive, thus it was maintained at all stages enclosed in a Kapton sealed capsule, custom-made for this experiment. The experiment was performed at beamline ID12, at T = 7 K, and by varying the applied field. Since XMCD is an element selective technique, any contribution caused by other elements could be disregarded, and a non-zero signal may be assigned to the Pt itself. Previous EXAFS experiments have determined that the structure of the Pt13 clusters is compatible with an icosahedral shape, with a central Pt atom surrounded by 12 others (see Figure 82). The cluster has a very large surface to volume ratio, and fits closely to the zeolite cage. In fact, only one out of 25 cages in the zeolite is occupied by Pt13 clusters.
 |
|
Fig. 82: Left: NaY zeolite. Right: Pt13 icosahedral cluster. |
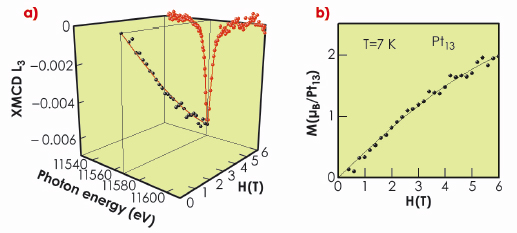
The XMCD spectra at the L2 and L3 edges were measured for T = 7 K and a maximum applied field of 6 Tesla (Figure 83a). The magnetic moment determined by the sum rule analysis is much larger than the Pauli paramagnetic contribution at that field, so it is proven that the nanoparticle supports a non-zero moment. In fact, the ratio of orbital to spin moment is as high as 0.32; i.e. more than 25% of the magnetic moment is of orbital origin. It can be attributed both to the high spin-orbit coupling of Pt and to surface effects such as Pt 5d quasiband narrowing, due to the breaking of symmetry of the Pt at the surface of a Pt13 cluster when compared to Pt in the bulk.
The XMCD at the energy of the L3 peak was followed as a function of applied field (Figure 83a), and found to behave as a superparamagnetic particle with a moment of 3.7 Bohr Magneton (Figure 83b). Only about 15 to 20% of the Pt13 particles contribute to the XMCD signal. The zeolite may interact with the Pt13 particles, exchanging electrons and thus filling the available holes in the 5d band, thus rendering a good fraction of the particles non-magnetic.
 |
|
Fig. 83: Fig. 83: a) Normalised isotropic Pt L2,3-edge XMCD spectra of the Pt13 sample at H = 6 T ( |
To conclude, we have demonstrated that size effects on the electronic quasiband structure of a Pt13 cluster may induce a non-zero magnetic moment on Pt. Under the current assumption that the magnetic moment has its origin in unpaired electrons, the total spin of the particle is in the range S ~ 2, with an orbital moment contribution of 25%. This is the first time that such a large orbital moment has been reported on a Pt13 particle.
References
[1] J. Bartolomé, L.M. García, F. Bartolomé, F. Luis, F. Petroff, C. Deranlot, F. Wilhelm and A. Rogalev, J. Magn. & Magn. Mat. 316, e9-e12 (2007).
[2] X. Liu, M. Bauer, H. Bertagnolli, E. Roduner, J. van Slageren and F. Phillipp. Phys. Rev. Lett. 97, 253401 (2006); X. Liu, M. Bauer, H. Bertagnolli, E. Roduner and J. van Slageren, F. Phillipp, Phys. Rev. Lett. 102, e049902 (2009).
Principal publication and authors
J. Bartolomé (a), F. Bartolomé (a), L.M. García (a), E. Roduner (b), Y. Akdogan (b), F. Wilhelm (c) and A. Rogalev (c), Physical Review B 77, 184420 (2008).
(a) Instituto de Ciencia de Materiales de Aragón, and Dpto. Física de la Materia Condensada, CSIC-Universidad de Zaragoza (Spain)
(b) Institut für Physikalische Chemie, Universität Stuttgart (Germany)
(c) ESRF



