- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Electronic structure and magnetism
- Redox-dependant sulphate coordination of neptunium in aqueous solutions
Redox-dependant sulphate coordination of neptunium in aqueous solutions
Neptunium is a by-product of nuclear energy generation. 237Np accumulates during the reactor operation time and later in the nuclear waste repository in large quantities. Neptunium is considered to be one of the most problematic actinide elements for nuclear waste storage due to its rich redox chemistry combined with a relatively high solubility in aqueous solution [1]. Although the redox phenomena have been investigated for several decades, the underlying mechanisms and the coordination of the species are not well known. Extended X-ray absorption fine structure (EXAFS) spectroscopy, in combination with electrochemistry, is a powerful tool to determine the complex structure of Np species in aqueous sulphate solution as a function of the redox conditions. The experiments were performed at the Rossendorf Beamline (BM20), the only beamline at the ESRF dedicated to studies of actinides in solution.
The samples were prepared by dissolving NpVIO2(ClO4)2·nH2O in aqueous solutions of sulphate, to give an Np concentration of 0.05 M. Different neptunium oxidation states such as Np4+, Np5+ and Np6+ were generated by potentiostatic electrolysis. Np L3-edge EXAFS measurements were carried out in transmission mode using a Si(111) double-crystal monochromator, and two Pt-coated mirrors for rejection of higher harmonics.
 |
|
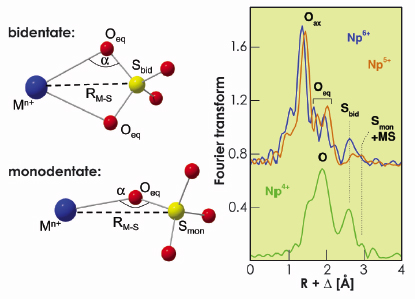
Fig. 102: a) Coordination mode of sulphate. b) Fourier Transforms of the Np L3-edge EXAFS spectra of 0.05 M Np sulphate species in aqueous solution of 2.0 M (NH4)2SO4. |
Sulphate is able to coordinate neptunium either in monodentate or in bidentate coordination mode, as shown in Figure 102. Both modes can be differentiated by determining the distances between neptunium and sulphur atoms, RNp-S, because sulphur is a relatively heavy backscatterer, easily detectable with EXAFS. The sulphate in bidentate coordination shows a Np-Sbid distance of ~3.1 Å, whereas monodentate sulphate shows a Np-Smon distance of ~3.6 Å. At oxidation states V and VI, the complex structure is similar, which can be seen from the small changes in the spectra plotted on top of each other in Figure 102. Neptunium in these oxidation states forms trans-dioxo cations NpO2n+ (n=1 and 2 for Np5+ and Np6+, respectively). The two double-bond oxygens, commonly called axial oxygens (Oax), do not easily form bonds with ligands. The sulphate coordination is hence restricted to the oxygen atoms in the equatorial plane (Oeq). The Np-Oax bond length decreases from 1.83 Å (Np5+) to 1.76 Å (Np6+), indicative of a bond strength enhancement with increasing charge of neptunium. In non-complexing media the redox reaction is restricted to a fully reversible electron transfer according to NpO25+ + e- ![]() NpO2+. In complexing media this redox reaction can be disturbed by side reactions in the equatorial plane. This latter situation occurs by the sulphate coordination: the cyclovoltammogram shows a non-reversible character of the Np5+/Np6+ redox couple at ~ 0.8 V (Figure 103). The EXAFS measurements show that bidentate sulphate coordination prevails at the hexavalent oxidation state, whereas for Np5+ the complexation is weaker and forms both mono- and bidentate sulphate complexes. The cyclovoltammogram shows also, that the Np4+/Np5+ redox couple, occurring at ~ –0.4 V, is fully irreversible. The EXAFS spectrum of Np4+ indicates the loss of the axial oxygens and the formation of a quasi-spherical shell of oxygen atoms. These oxygen atoms belong to sulphate groups which are again predominantly bidentately coordinated.
NpO2+. In complexing media this redox reaction can be disturbed by side reactions in the equatorial plane. This latter situation occurs by the sulphate coordination: the cyclovoltammogram shows a non-reversible character of the Np5+/Np6+ redox couple at ~ 0.8 V (Figure 103). The EXAFS measurements show that bidentate sulphate coordination prevails at the hexavalent oxidation state, whereas for Np5+ the complexation is weaker and forms both mono- and bidentate sulphate complexes. The cyclovoltammogram shows also, that the Np4+/Np5+ redox couple, occurring at ~ –0.4 V, is fully irreversible. The EXAFS spectrum of Np4+ indicates the loss of the axial oxygens and the formation of a quasi-spherical shell of oxygen atoms. These oxygen atoms belong to sulphate groups which are again predominantly bidentately coordinated.
 |
|
Fig. 103: Cyclic voltammograms of 0.05 M Np(VI) in an aqueous solution of 2.0 M (NH4)2SO4 at pH 1.1, Au working electrode, start potential: 1.3 V, initial scan direction: cathodic, scan rate: 400 mV/s. The sulphate coordination of the Np5+, Np4+ and Np6+ solution species is shown as ball and stick drawing. |
The combined EXAFS and cyclovoltammetry data reveal an unexpected pattern of sulphate coordination and redox behaviour: although the actinyl ion NpO2n+ remains preserved in the redox reaction between Np5+ and Np6+, the sulphate coordination in the equatorial plane changes. This results in a deviation from the usual reversible redox behaviour. In contrast, the irreversible nature of the transition from Np5+ to Np4+ is expected due to the loss of the axial oxygen atoms and the complete replacement of water ligands by sulphate. This example demonstrates that EXAFS is able to detect the reaction mechanisms of quasi-reversible and even irreversible redox reactions.
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft, Contract HE2297/2-2.
Reference
[1] Z. Yoshida, S. G. Johnson, T. Kimura, J. R. Krsul, in The Chemistry of the Actinide and Transactinide Elements (3rd Ed.), L. R. Morss, N. M. Edelstein, J. Fuger (Eds.), Springer, 699 (2006).
Principal publication and authors
C. Hennig (a), A. Ikeda-Ohno (a,b), S. Tsushima (a) and A.C. Scheinost (a), Inorg. Chem. 48, 5350 (2009).
(a) Institute for Radiochemistry, Forschungszentrum Dresden-Rossendorf (Germany)
(b) Synchrotron Radiation Research Center, Japan Atomic Energy Agency (Japan)



