- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- X-ray imaging
- Characterisation of a degraded cadmium yellow (CdS) pigment in an oil painting by means of synchrotron radiation based X-ray techniques
Characterisation of a degraded cadmium yellow (CdS) pigment in an oil painting by means of synchrotron radiation based X-ray techniques
Cadmium sulphide exists in two crystalline and one amorphous form. The hexagonal form (![]() -CdS) is found in nature as the mineral greenockite while the cubic form (ß-CdS) is called hawleyite; greenockite is frequently used as yellow pigment in modern paints. Manufacturers started to make intense use of this pigment from the moment cadmium became commercially available as a base material (around the 1840’s). This popularity was mainly due to the pigment’s high tinting and covering power, bright yellow colour, wide applicability (artists’ paint, metallurgy, ceramics, medical use, etc.) and suitability for mass production. In addition, at the beginning of the 20th century, CdS was thought to be highly stable in oil paint and water colours, which was not the case for chrome yellow (PbCrO4), the only bright yellow alternative available for painting at that time. Consequently, prominent 19th-20th century painters such as Claude Monet, Vincent Van Gogh and Pablo Picasso frequently employed CdS, as was amply documented by earlier analytical research. Yet, in spite of its excellent reputation with regards to permanency, fading of the yellow colour of CdS and loss of adhesion of the oil paint has been reported.
-CdS) is found in nature as the mineral greenockite while the cubic form (ß-CdS) is called hawleyite; greenockite is frequently used as yellow pigment in modern paints. Manufacturers started to make intense use of this pigment from the moment cadmium became commercially available as a base material (around the 1840’s). This popularity was mainly due to the pigment’s high tinting and covering power, bright yellow colour, wide applicability (artists’ paint, metallurgy, ceramics, medical use, etc.) and suitability for mass production. In addition, at the beginning of the 20th century, CdS was thought to be highly stable in oil paint and water colours, which was not the case for chrome yellow (PbCrO4), the only bright yellow alternative available for painting at that time. Consequently, prominent 19th-20th century painters such as Claude Monet, Vincent Van Gogh and Pablo Picasso frequently employed CdS, as was amply documented by earlier analytical research. Yet, in spite of its excellent reputation with regards to permanency, fading of the yellow colour of CdS and loss of adhesion of the oil paint has been reported.
 |
|
Fig. 140: a) Optical photograph of the oil painting ‘Still life with Cabbage’ by James Ensor (ca.1921, KM 105.303); b) detail of the exposed yellow paint surface (X40) showing white globules; c) Detail of the right-lower corner of the painting: the yellow paint covered by the frame retains its vivid yellow colour while the paint in the exposed areas has become dull due to the formation of the white globules. |
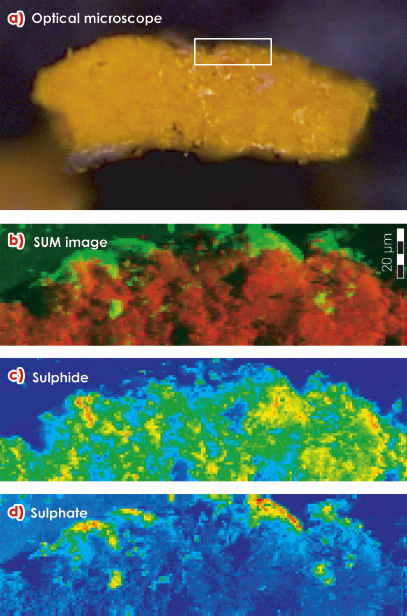
On several paintings by James Ensor (1860-1949), an avant-garde painter, a gradual fading of originally bright yellow CdS areas is observed. This phenomenon is associated with the formation of small white-coloured globules on top of the original paint surface. Figure 140 shows Ensor’s painting “Still Life with Cabbage” from which small amounts of yellow paint and the whitish material were examined at the ESRF. Microscopic X-ray absorption near edge spectroscopy (µ-XANES) experiments at ID21 were used to demonstrate that sulphur, originally present in sulphidic form (S2-) is oxidised during the transformation to the sulphate form (S6+). The presence of cadmium sulphate (CdSO4.2H2O) and ammonium cadmium sulphate ((NH4)2Cd(SO4)2) at the surface was confirmed by microscopic X-ray diffraction measurements at ID18F. The latter salt is suspected to result from a secondary reaction of cadmium sulphate with ammonia. The Cd itself remains in its original oxidation state (Cd2+). S-chemical state maps recorded from cross-sections reveal that during the last 100 years, the oxidation front has penetrated into the yellow paint up to ca. 1-2 micrometres (Figure 141).
 |
|
Fig. 141: a) Optical micrograph of a cross section of partially degraded yellow paint. White rectangle: area where S-chemical state maps (b-d) were obtained. b) Red-green composite of (c) and (d), red: sulphides and green: sulphates; c) sulphide distribution; d) sulphate distribution. Map size = 184 x 50 µm2; step size = 1 x 1 µm2. |
The superficial deterioration was exclusively detected in areas where the paint was directly exposed to a combination of light and humidity, allowing the following photo-induced reaction to take place:
CdS + 2O2 + H2O ![]() CdSO4.H2O
CdSO4.H2O
The formation of the globules can be attributed to a process of recurring moistening and drying of the paint surface that induces the repeated dissolution and (re)precipitation at and near the surface of the highly hygroscopic cadmium-sulphate; fluctuating climatic conditions in the museum gallery are likely to drive this process.
To prevent further progression of the oxidation process and loss of colour-vividness, the amount of UV radiation reaching the painting surface must be limited while care must be taken to stabilise the relative humidity level of the surrounding atmosphere.
Principal publication and authors
G. Van der Snickt (a), J. Dik (b), M. Cotte (c,d), K. Janssens (a), J. Jaroszewicz (a), W. De Nolf (a), J. Groenewegen (b) and L. Van der Loeff (e), Anal. Chem. 81, 2600 (2009).
(a) University of Antwerp (Belgium)
(b) Delft University of Technology (The Netherlands)
(c) C2RMF (France)
(d) ESRF
(e) Kröller-Müller Museum (The Netherlands)



