- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- Detection of deviation from ideal solution behaviour in Zr-Cu metallic glasses with addition of Al
Detection of deviation from ideal solution behaviour in Zr-Cu metallic glasses with addition of Al
Metallic glasses are metastable materials with unusual structures and remarkable properties. They are made by carefully cooling liquid alloys so that they solidify without crystallising. Thanks to an irregular, tightly packed internal arrangement of atoms, metallic glasses are mouldable like plastic when heated to the glass transition temperature, yet stronger than typical crystalline alloys at room temperature [1].
The use of synchrotron X-rays can be highly advantageous for analysing the structure of metallic glasses. Conventional X-ray beams are completely absorbed near the surface, whereas high energy, high flux radiation penetrates even thick samples, providing a deeper understanding of the unique internal structure of metallic glasses.
Using real space pair distribution functions (PDFs) derived from high precision X-ray diffraction data collected at beamline ID11, we attempt to probe the internal structure of metallic glasses based on Zr and Cu and investigate the effect of a third element (Al) on their atomic structure. The results provide a guide for producing thicker alloy glasses with enhanced properties.
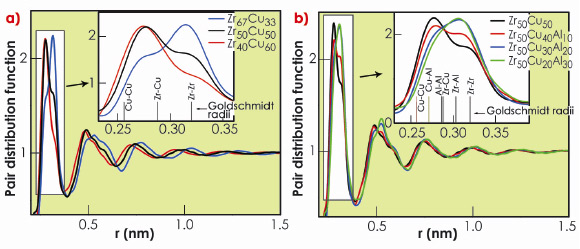
The PDFs of the Zr-Cu binary metallic glasses (Figure 46a) reveal how the atomic structure changes with the Zr/Cu atomic ratio. Increasing Cu content generates significant differences in the PDFs, suggesting modifications in the short (SRO) and medium range order (MRO) up to 1.5 nm. As expected, the structural changes in the nearest neighbour (nn) shell (inset Figure 46a), indicate an increased number of Zr-Zr atomic bonds for the Zr-rich compositions, and an increased number of Cu-Zr and Cu-Cu atomic bonds for the Cu-rich site.
 |
|
Fig. 46: Pair distribution functions for metallic glasses: a) Zr-Cu binary and b) Zr-Cu-Al ternary. Insets: close-up of the first PDF peak. |
Addition of Al to the Zr-Cu metallic glasses leads to significant modifications of SRO and MRO, Figure 46b. The nn shell (inset Figure 46b) indicates the formation of a significant number of Zr-Al atomic bonds. This behaviour can be attributed to strongly attractive Zr-Al atomic interactions consistent with the highly negative heat of mixing ![]() Hmix of –44 kJ mol–1 which is twice that of Zr-Cu (–23 kJ mol–1), whereas
Hmix of –44 kJ mol–1 which is twice that of Zr-Cu (–23 kJ mol–1), whereas ![]() Hmix for Cu-Al is negligible (–1 kJ mol–1). Al has an intermediate atomic size between those of Zr and Cu atoms. Because of the strongly negative heat of mixing between Zr and Al atoms, addition of Al promotes chemical short range ordering in the liquid, improves the local packing efficiency and slows down long range atomic diffusion required for crystallisation, leading to increased glass forming ability.
Hmix for Cu-Al is negligible (–1 kJ mol–1). Al has an intermediate atomic size between those of Zr and Cu atoms. Because of the strongly negative heat of mixing between Zr and Al atoms, addition of Al promotes chemical short range ordering in the liquid, improves the local packing efficiency and slows down long range atomic diffusion required for crystallisation, leading to increased glass forming ability.
For an ideal solution, the total PDF for Zr-Cu binary alloys would be correlated with the partial PDF of Zr-Zr, Zr-Cu, and Cu-Cu atomic pairs according to the following relation:
![]()
Approaching the ternary ZrCuAl alloys as ideal solutions, the correlation between total and partial PDFs would be given as:
![]()
where Wij= CiCjFiFj/(![]() CiFi)2, Wij are the ideal solution weight factors, Ci are the atomic concentrations and Fi are the atomic form factors.
CiFi)2, Wij are the ideal solution weight factors, Ci are the atomic concentrations and Fi are the atomic form factors.
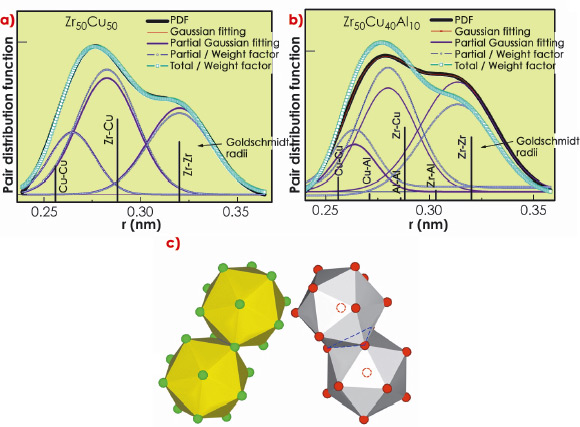
The PDFs derived from experimental XRD results for Zr-Cu binary metallic glasses were found to be in good agreement with those expected from the weight factors of eq. 1 (Figure 47a), indicating that the Zr-Cu metallic glass can be satisfactorily approximated as an ideal solid solution. Because of the weak interactions between Zr-Cu atoms in the binary alloy, only very thin glasses can be cast from Zr-Cu liquid alloys. However, adding a third strongly interacting component, Al, to the liquid alloy leads to a stronger glass that can be cast at thicknesses of up to several millimetres.
 |
|
Fig. 47: Gaussian fitting of the first PDF peak for a) Zr50Cu50 and b) Zr50Cu40Al10 metallic glasses. Experimental PDF (thick black curves); calculated partial PDFs derived from the experimental PDF (thin red curves); Gaussian partial PDFs scaled to the weight factors of an ideal solution (thin blue discontinuous curves); expected total PDFs based on the weight factors (thick discontinuous light blue curves). c) Schematic diagram showing how the atoms in Zr-Cu based metallic glasses form icosahedral clusters that pack tightly together by sharing edges (left) or faces (right) [2]. |
We have therefore determined that in contrast to the binary Zr-Cu, ternary Zr-Cu-Al metallic glasses deviate markedly from ideal solution behaviour (Figure 47b). Zr-Cu-Al glasses are comprised of finite regions of atomic order, providing the extra support needed to form thicker glasses. This phenomenon is believed to be due to attractive interactions between the sp electrons of Al and the d-electron shell of the larger Zr atoms. The atoms arrange in clusters resembling icosahedra, which pack together tightly in the glass (Figure 47c), as observed in a previous study [2].
References
[1] A.R. Yavari, Nature 439, 405 (2006).
[2] Ch.E. Lekka, A. Ibenskas, A.R. Yavari and G.A. Evangelakis, Appl. Phys. Lett. 91, 214103 (2007).
Principal publication and authors
K. Georgarakis (a,b), A.R. Yavari (b,a,c), D.V. Louzguine-Luzgin (a), J. Antonowicz (d), M. Stoica (e), Y. Li (b), M. Satta (b), A. LeMoulec (b), G. Vaughan (c) and A. Inoue (a), Applied Physics Letters 94, 191912 (2009).
(a) WPI-AIMR, Tohoku University, Sendai (Japan)
(b) Euronano-SIMaP-CNRS, INP Grenoble, St-Martin-d’Hères (France)
(c) ESRF
(d) Faculty of Physics, Warsaw University of Technology (Poland)
(e) IFW Dresden, Institute for Complex Materials (Germany)



