- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Soft condensed matter
- Tracking global conformational changes in a membrane protein by time-resolved wide-angle X-ray scattering
Tracking global conformational changes in a membrane protein by time-resolved wide-angle X-ray scattering
Bacteriorhodopsin and proteorhodopsin are the simplest known light-driven proton pumps. Following the absorption of a photon by a buried retinal molecule bound via a protonated Schiff base to a conserved lysine residue of helix G, a sequence of conformational changes occur within these integral membrane proteins that drive proton pumping. Bacteriorhodopsin has been intensely studied for almost four decades using numerous biophysical techniques. On the other hand, proteorhodopsin, a homologue found in ocean bacteria, was discovered only a decade ago, but it is now recognised that this bacteriorhodopsin homologue provides a major primary energy input into the ocean’s biosphere.
Several X-ray diffraction studies of the resting state and trapped intermediate states of bacteriorhodopsin have been reported [1]. Although many details concerning the movements of amino acid residues and water molecules were revealed in these earlier intermediate trapping studies performed at low-temperature, these results were somewhat controversial since the nature and magnitude of the observed a-helix movements did not always agree. Time-resolved wide-angle X-ray scattering (WAXS) is a method that has potential to resolve the nature and amplitudes of large-scale structural rearrangements occurring in proteins at room temperature, and to characterise the timescales over which these movements occur. Although the method was originally developed at beamline ID09 for time-resolved studies of structural changes occurring in small-molecules in solution [2], this approach was recently extended to study protein conformational dynamics using hemoglobin in complex with carbon-monoxide as a proof-of-principle [3].
 |
|
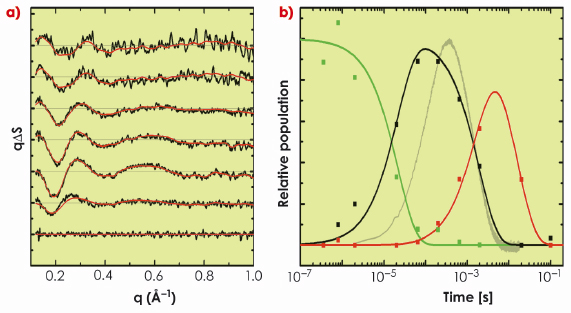
Fig. 67: a) Time-resolved WAXS difference data recorded from bacteriorhodopsin following photo-activation, and b) its decomposition into three components (early, green; intermediate, black; late, red). Solid red lines in the left panel show how this spectral decomposition accurately represents the experimental data (black lines). The transition from the early (green) to the intermediate (black) conformational state precedes the primary proton transfer in the bacteriorhodopsin photocycle (grey line, right panel). |
In a collaborative study involving groups in Gothenburg, Uppsala and the ESRF, we applied time-resolved WAXS to probe the structural dynamics of the light-driven proton pumps of bacteriorhodopsin and proteorhodopsin. As shown in Figure 67, difference WAXS data recorded from bacteriorhodopsin could be decomposed into three components, and permits elucidation of the timescales upon which these components rise and fall. Moreover, structural refinement against the difference WAXS data revealed that significant movements of the cytoplasmic halves of a-helices E and F, and of the extracellular half of helix C, occur prior to the primary proton transfer event from the retinal Schiff base to Asp85. The amplitudes of these movements were observed to increase a further 50% during the latter half of the photocycle after the Schiff base was deprotonated (Figure 68). This overall picture provides a significantly simpler description of the structural changes that occur during proton pumping by bacteriorhodopsin than emerged from intermediate trapping studies. Moreover, very similar structural conclusions were drawn from time-resolved WAXS data recorded from proteorhodopsin, revealing shared dynamical principles for proton pumping. Finally, by successfully resolving the nature and time-scales of a-helical movements that occur in a well characterised membrane protein like bacteriorhodopsin, this work opens the door to future studies of the structural dynamics of more complex integral membrane proteins.
 |
|
Fig. 68: Large-scale |
References
[1] R. Neutze, E. Pebay-Peyroula, K. Edman, A. Royant, J.Navarro and E.M. Landau, Biochim. Biophys. Acta 1565, 144-167 (2002).
[2] R. Neutze, R. Wouts, S. Techert, J. Davidsson, M. Kocsis, A. Kirrander, F. Schotte and M. Wulff, Phys. Rev. Lett. 87, 195508 (2001).
[3] M. Cammarata, M. Levantino, F. Schotte, P. A. Anfinrud, F. Ewald, J. Choi, A. Cupane, M. Wulff and H. Ihee, Nature Methods 5, 881-886 (2008).
Principal publication and authors
M. Andersson (a), E. Malmerberg (b), S. Westenhoff (b), G. Katona (b), M. Cammarata (c), A. B. Wöhri (a), L. C. Johansson (b), F. Ewald (c), M. Eklund (d), M. Wulff (c), J. Davidsson (d) and R. Neutze (b), Structure 17, 1265-1275 (2009).
(a) Department of Chemical and Biological Engineering, Chalmers University of Technology, Göteborg (Sweden)
(b) Department of Chemistry, Biochemistry and Biophysics, University of Gothenburg, Göteborg (Sweden)
(c) ESRF
(d) Department of Photochemistry and Molecular Science, Uppsala University (Sweden)



