- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Dynamics and extreme conditions
- Tetrahydrofuran clathrate hydrate formation
Tetrahydrofuran clathrate hydrate formation
Since the oil disaster in the Gulf of Mexico, clathrate hydrates have gained media attention. These ice-like compounds consist of water nano-cages in which guest molecules, mainly gases, can be trapped. Hydrates, containing various alkane molecules from the oil, formed at the steel caps that were intended to collect the oil leaking from the drill hole. These hydrates blocked the caps and made controlled pumping impossible. A similar problem is known from gas and oil pipelines, where hydrate formation has to be inhibited to suppress blockages. Further interest in hydrates is for those existing in the ocean floor which could be used as an energy source and others may be used for safe gas storage, e.g. hydrogen for fuel cells [1]. Tetrahydrofuran (THF)-hydrogen double hydrates are interesting because the presence of THF decreases the hydrate stability pressure by approximately two orders of magnitude [1]. A schematic diagram of THF hydrate is shown in Figure 22. Hydrate formation from the liquid phase at molecular length scales was mainly studied by theoretical work, research that is important for inhibition of hydrate formation in the oil industry. Furthering this knowledge is essential for other applications such as gas storage. Various hydrate formation models based on molecular dynamics (MD) simulations have been published in the literature [1], however, experimental data are still rare. In a previous study we found that the hydrate formation at water-CO2 interfaces follows a stochastical model instead of forming hydrate precursors in a supercooled liquid phase [2].
 |
|
Fig. 22: Schematic picture of the THF hydrate structure. The cage structure formed by the water molecules is represented as red-white sticks. Because the THF molecules only occupy the large cages of the sII hydrate structure [1], a second guest can be placed in the small cages. |
In contrast to the majority of hydrates that usually form at the water surface, THF hydrate forms from a liquid mixture of water and THF. Thus, bulk sensitive methods have to be used to study the THF hydrate formation process. We used X-ray Raman scattering at ID16 to measure the oxygen K-edge of a liquid mixture at room and supercooled (T<277 K) temperatures. X-ray Raman scattering is very sensitive to changes in the sample’s local structure [3]. In particular, the formation of hydrate precursors will influence the fine structure of the O K-edge. Furthermore, we measured spectra of THF hydrate at various temperatures to study a potential structural rearrangement, e.g. due to a strong guest-host interaction as suggested by MD simulations [4].
 |
|
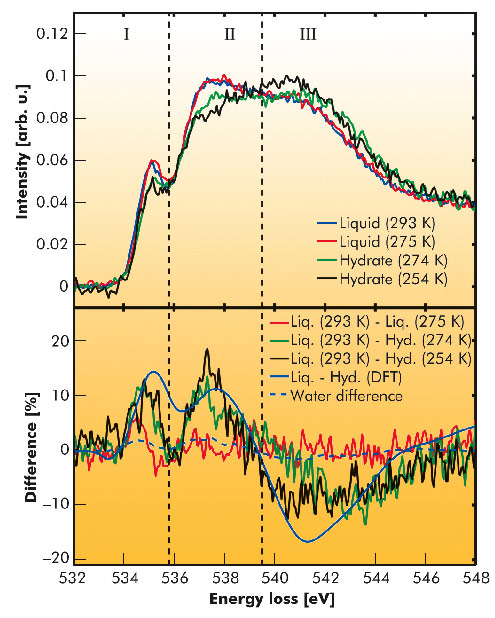
Fig. 23: X-ray Raman scattering spectra (top) and differences (bottom) of the liquid mixture and the hydrate. The data for water represents a difference between 295 K and 275 K [5]. |
The experimental spectra are presented in Figure 23 together with difference spectra referenced to the spectra of the liquid at room temperature. Apparently, the difference of the liquid phases is weak and in agreement with water spectra representing the temperature effect on the water’s hydrogen bond network [5]. Thus, the formation of hydrate precursors is highly unlikely. Moreover, the difference between the two hydrate spectra suggests a structural change in the hydrate with an increase of order at lower temperatures. This is supported by DFT calculations that yield a good agreement with the experimental data for the hydrate at 254 K. Hence, a rearrangement of the hydrate structure to a less ordered sample is suggested for elevated temperatures. Altogether, we were able to favour a stochastic hydrate formation model without occurrence of hydrate precursor clusters for THF hydrate as were also observed at the water-CO2 interface [3] and we found the first experimental evidence for a distorted THF hydrate structure at high temperatures.
Principal publication and authors
H. Conrad (a,b), F. Lehmkühler (a,b), C. Sternemann (a), A. Sakko (c), D. Paschek (d), L. Simonelli (e), S. Huotari (c,e), O. Feroughi (a), M. Tolan (a) and K. Hämäläinen (c), Phys. Rev. Lett., 103, 218301 (2009).
(a) Fakultät Physik/DELTA, TU Dortmund (Germany)
(b) HASYLAB at DESY, Hamburg (Germany)
(c) Department of Physics, University of Helsinki (Finland)
(d) Rensselaer Polytechnic Institute, Troy (USA)
(e) ESRF
References
[1] E.D. Sloan and C.A. Koh, Clathrate Hydrates of Natural Gases, CRC Press Inc. (2008).
[2] F. Lehmkühler, M. Paulus, C. Sternemann, D. Lietz, F. Venturini, C. Gutt and M. Tolan, J. Am. Chem. Soc. 131, 585 (2009).
[3] J. Tse, D.M. Shaw, D.D. Klug, S.Patchkovskii, G. Vankó and M. Krisch, Phys. Rev. Lett., 100, 095502 (2008), T. Pylkkänen, V.M. Girodano, J.C. Chervin, A. Sakko, M. Hakala, J.A. Soininen, K. Hämäläinen, G. Monaco and S. Huotari, J. Phys. Chem. B 114, 3804 (2010).
[4] S. Alavi, R. Susilo and J.A. Ripmeester, J. Chem. Phys., 130, 174501 (2009).
[5] U. Bergmann, D. Nordlund, Ph. Wernet, M. Odelius, L.G.M. Petterson and A. Nilsson, Phys. Rev. B., 76, 024202 (2007).



