- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Dynamics and extreme conditions
- Novel X-ray imaging technique with molecular-bond sensitivity
Novel X-ray imaging technique with molecular-bond sensitivity
X-ray spectroscopic imaging techniques can study the distribution of elements and chemical compounds inside a material. For this purpose X-ray images can, for example, be taken by varying the energy of monochromatic X-rays in the vicinity of the elements’ characteristic absorption edges. This is feasible for elements belonging to at least the third row of the periodic table since only then the required X-ray energy is high enough to probe matter on macroscopic length scales. But what if one would like to study lighter elements, such as carbon and oxygen? The corresponding X-rays are rather soft and probe matter only at a depth of a few micrometres. Even though such chemical imaging can be done [1], there are severe constraints for samples to be studied in three dimensions. Hard X-rays (from 10 keV up) are capable of probing materials in three dimensions over macroscopic length scales, so would it be possible to use them to study the chemistry of light elements? A new technique has now been developed at ID16 to do exactly that. The principle is based on inelastic X-ray scattering (IXS) [2].
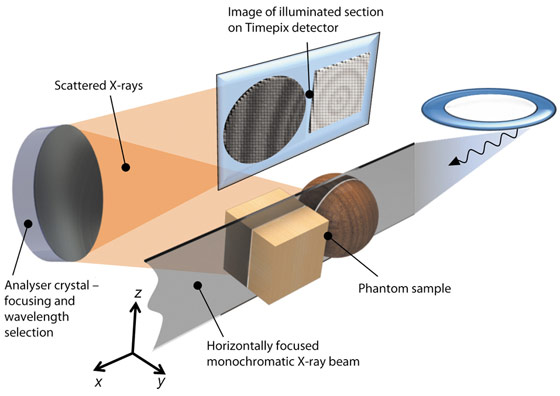
IXS probes electron excitations by measuring the energy-loss spectra of hard X-rays scattered by electrons. In the experiment, the sample is placed in a monochromatic X-ray beam focused in one dimension (Figure 22). The X-rays pass through the sample illuminating a slice-shaped region, the thickness of which is determined by the incident beam size. Some of the X-rays will be scattered by electrons within the illuminated section. The scattered X-rays are collected by a spherically curved crystal analyser, which reflects and focuses them onto a two-dimensional detector. The experimenter thus sees an image that reveals, instead of the density of the sample, the distribution of electron excitations within it. If the sample is translated in the horizontal direction, one can collect these section images from different positions within the sample, and the slices can be stacked together to form a three-dimensional model. The spatial resolution is limited by the beamline optics and is at present in the 100 µm range, but will be improved to ~10 µm at the forthcoming UPBL6 upgrade beamline.
The analyser is the heart of the experiment. It is actually a focusing monochromator, and reflects only those scattered photons that fall into a certain energy bandwidth (about 0.5 eV at 10 keV). While analyser crystals have been used in the IXS experiments for decades, their imaging capabilities have not been fully exploited before. To achieve this, the ID16 team has developed new technologies to optimise analysers for imaging. The IXS spectra are measured by changing the incident X-ray beam energy, and spectra are recorded for each pixel of the illuminated section.
 |
|
Fig. 22: Schematics of the setup. A phantom sample is represented by an ensemble of a sphere and a cube. The analyser reflects scattered photons, producing a mirror image of a two-dimensional section of the sample on the pixel detector. |
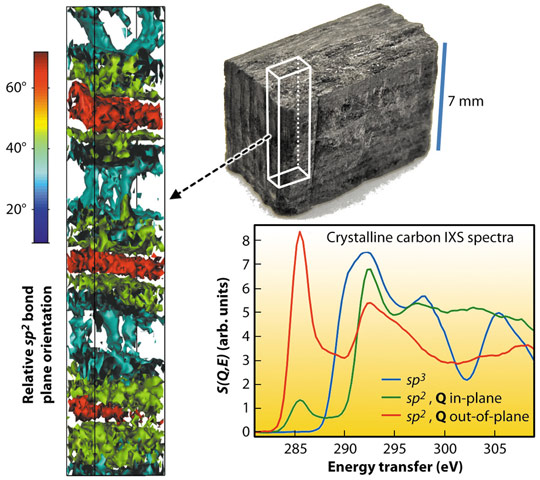
One can use the technique to determine for instance local molecular bond variations in carbon composite materials. Figure 23 shows the application of the method to a carbon fibre reinforced silicon carbide sample as an example of a materials science characterisation. A typical example of an IXS excitation is that of a deeply-bound core electron, in which case IXS spectra are comparable to those of X-ray absorption spectroscopy. Therefore, from the IXS spectra of carbon 1s core electrons, different carbon bonds are easily discernible. Each measured voxel contains an IXS spectrum that reveals the average carbon bond type within it. From the sample shown in Figure 23, we determined the carbon bond distribution, and found crystalline sp2 dominating. Since the sp2 bond is anisotropic, the spectra are also sensitive to its orientation, revealing a periodically alternating bond orientation.
 |
|
Fig. 23: Application of the technique to a carbon composite sample. |
In conclusion, it is now possible to study the three-dimensional distribution and chemistry of light elements in bulk samples and in confined environments. This is achieved by using novel contrast mechanisms for three-dimensional imaging such as IXS. Examples of potential research objectives are batteries, fuel cells, and rare geological samples. This should open up new perspectives in materials science, chemistry, physics and geology, once the IXS Upgrade beamline UPBL6 becomes operational.
Principal publication and authors
S. Huotari (a,b), T. Pylkkänen (a,b), R. Verbeni (a), G. Monaco (a) and K. Hämäläinen (b), Nature Mater. 10, 489-493 (2011).
(a) ESRF
(b) University of Helsinki (Finland)
References
[1] G.A. Johansson, T. Tyliszczak, G.E. Mitchell, M.H. Keefe and A.P. Hitchcock, J. Synchrotron Rad. 14, 395-402 (2007).
[2] Electron Dynamics by Inelastic X-Ray Scattering, W. Schülke, Oxford University Press, Oxford (2007).



