- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Dynamics and extreme conditions
- Silicon carbonate formed from carbon dioxide and silicon dioxide
Silicon carbonate formed from carbon dioxide and silicon dioxide
CO2 and SiO2 are two archetypal group IV oxides of paramount importance for chemistry and planetary sciences. The chemical relationship between these substances, in particular their reactivity, is of great interest. Although both systems are group IV oxides, they are remarkably different under ambient conditions: CO2 is molecular and is held together by C=O double bonds, while SiO2 forms network structures involving Si-O single bonds. These bonding patterns radically change under pressure. CO2 has been found to form non-molecular solid phases above 30 GPa similar to SiO2 [1]. A possible approach to favour the chemical reaction between CO2 and SiO2 is to select a micro-porous silica polymorph, such as silicalite. At ambient conditions, silicalite is characterised by a framework of four-, five, six- and ten-membered rings of SiO4 tetrahedra with 5.5 Å pores that can be completely filled by CO2 under pressure [2] (Figure 18, inset). The choice of silicalite was motivated by the large effective surface exposed to the CO2 in the pores, which is a crucial factor for enhancing the chemical reaction.
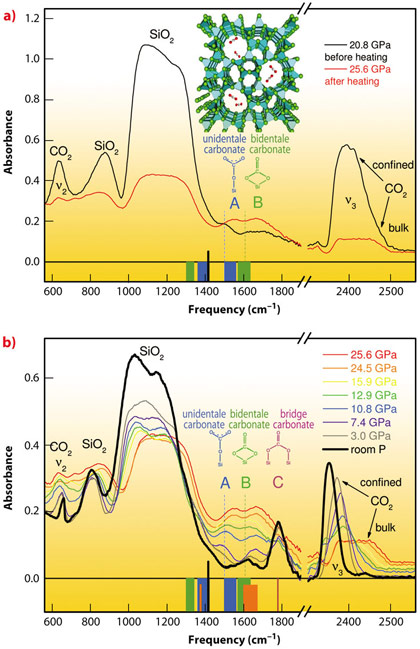
We examined chemical reactions between silicalite SiO2 and CO2, by compressing mixed samples at 18-26 GPa and subsequent heating at 600-740 K. In the infra-red (IR) spectrum of the temperature-quenched material (Figure 18a) the peaks of silicalite are remarkably reduced and the peaks of micro-confined CO2 almost completely vanished. At the same time, two new peaks appeared, labelled A and B, that belong neither to molecular CO2 nor to silicalite. Therefore, the most fundamental aspect of a binary chemical reaction is demonstrated: two substances, silicalite and CO2, react with each other, and a product is formed, identified by peaks A and B. Upon lowering pressure the new peaks gradually disappear below 15 GPa, and a new, non-molecular peak, C, emerges. In parallel, we observe the formation of micro-confined CO2 and the intensification of the silicalite peaks again (Figure 18b). Finally, at room pressure, peak C disappears after a few days along with the peak of residual confined CO2, thereby showing the overall reversibility of the transformation. The new IR peaks were assigned to C-O stretching modes of unidentate, bidentate and bridged silicon carbonate species.
 |
|
Fig. 18: IR spectra of mixed silicalite and CO2 showing the formation of silicon carbonate. The new compound is identified by the peaks A, B and C, assigned to unidentate, bidentate and bridged silicon carbonate species. Inset: silicalite structure. CO2 fills the micro-pores of the zeolite under pressure, and reacts with the silica framework upon heating. |
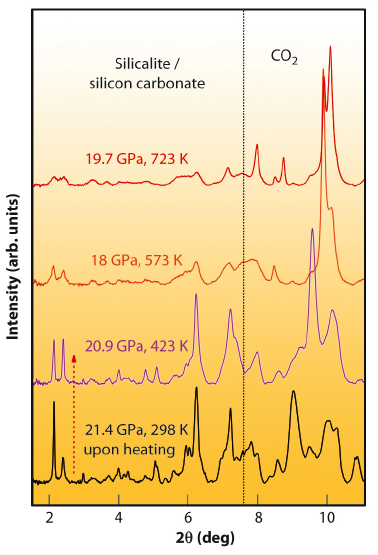
As an additional proof of the chemical reaction and of the nature of the compound, we measured the X-ray diffraction patterns (Figure 19) on a sample compressed to 21.4 GPa and then heated to 723 K at beamline ID27. The Bragg peaks of orthorhombic silicalite progressively broaden upon increasing temperature. This is a clear indication of the chemical reaction, as diffraction peaks should instead sharpen because of the usual temperature enhancement of the crystal quality and relaxation of any deviatoric stress. This also shows that the chemical reaction takes place progressively, ending up in a product that is a highly-strained crystal still exhibiting the structure of the original silicalite. This is not unexpected, since the carbonates form at the micro-pore surface, which in turn does not alter the pore arrangement within the unit cell, but affects the long range periodicity of the structure. The carbonate groups form in a random manner without any long-range order. They can also be expected to induce local geometrical distortions to the framework.
 |
|
Fig. 19: High pressure X-ray diffraction patterns of the silicalite-CO2 mixture, showing the formation of a highly-strained, disordered crystal upon heating. At angles higher than the dashed vertical line the pattern is dominated by peaks of different molecular phases of bulk CO2. |
The data presented here consistently shows that SiO2 and CO2 undergo high P-T chemical reactions of the type: xSiO2+yCO2 ![]() SixCyO(2x+2y), that result in the formation of one or more silicon carbonate compounds. Although the reaction occurs at the surface of the silicalite micropores, the final product has the nature of a real bulk compound due to the particular structure of zeolitic silicalite. In fact, all of the tetrahedra in silicalite are on the surface of the micro-pores, thus a surface reaction can involve, in principle, all the SiO2 as would be the case in a bulk reaction. However, the silicalite framework is retained, although highly strained, and the product is thus a non-stoichiometric silicon carbonate.
SixCyO(2x+2y), that result in the formation of one or more silicon carbonate compounds. Although the reaction occurs at the surface of the silicalite micropores, the final product has the nature of a real bulk compound due to the particular structure of zeolitic silicalite. In fact, all of the tetrahedra in silicalite are on the surface of the micro-pores, thus a surface reaction can involve, in principle, all the SiO2 as would be the case in a bulk reaction. However, the silicalite framework is retained, although highly strained, and the product is thus a non-stoichiometric silicon carbonate.
Principal publication and authors
M. Santoro (a,b), F.A. Gorelli (a,b), J. Haines (c), O. Cambon (c), C. Levelut (d) and G. Garbarino (e), Proc. Natl. Acad. Sci. U.S.A. 108, 7689-7692 (2011).
(a) European Laboratory For Non Linear Spectroscopy (LENS), Firenze (Italy)
(b) IPCF-CNR, Roma (Italy)
(c) Institut Charles Gerhardt Montpellier, Equipe Chimie et Cristallochimie des Matériaux, UMR 5253, CNRS, UM2, Montpellier (France)
(d) Laboratoire Charles Coulomb, UMR 5221, CNRS, UM2, Montpellier (France)
(e) ESRF
References
[1] M. Santoro and F.A. Gorelli, Chem. Soc. Rev. 35, 918-931 (2006).
[2] J. Haines et al., J. Am. Chem. Soc. 132, 8860-8861 (2010).



