- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Electronic structure and magnetism
- Molecular near-infrared to visible light upconversion
Molecular near-infrared to visible light upconversion
Near-Infrared to visible photon up-conversion is a process in which photoexcitation at a certain wavelength in the near infrared is followed by luminescence at a shorter wavelength in the visible. In this process, low energy photons are “converted” to higher energy photons. This phenomenon could be of potential use in liquid solar cell applications in order take advantage of the infrared part of the solar spectrum or in biological imaging to design visible emitting bio-luminescent probes which are excited with infrared radiation. Using a longer excitation wavelength than in the visible range, infrared presents several advantages such as less phototoxicity and a deeper penetration depth into living tissues. Moreover, compared to classical fluorescence spectroscopy, up-conversion microscopy does not suffer from noise due to the auto-luminescence of the sample [1].
 |
|
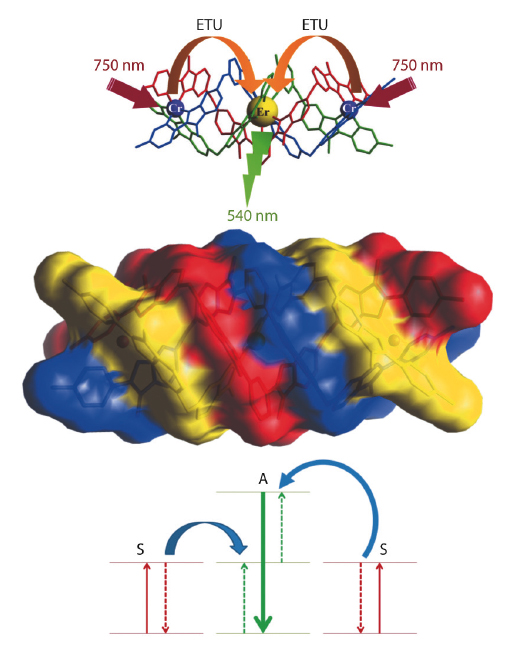
Fig. 95: The molecular triple-metallic complexes with the three metal atoms aligned and wrapped by three helical ligands. The simplified energy diagram shows the energy transfer between the two chromium atoms (sensitiser) and the erbium atom (acceptor) for the proposed ETU mechanism. |
The minimum prerequisite for the generation of upconversion luminescence by a material is the existence of two metastable excited states, the first serving as an excitation reservoir, and the second as the emitting state. Different possible processes of up-conversion have been described [2], the two most common being excited-state absorption (ESA) and energy transfer upconversion (ETU). ESA is a single ion mechanism which involves two sequential absorption processes. In the ETU process, at least two closely spaced ions are required. A sensitiser, which is excited to an intermediate excited state, transfers its energy to a neighbouring chromophore called acceptor, also in an intermediate excited state, promoting it to the upper emitting level (Figure 95). The existence of several excited states located between the ground state and the target excited state in lanthanide complexes makes them good candidates for such an upconversion mechanism, although it has long been thought that the vibrational oscillation of the organic ligands would quench this process for trivalent lanthanide ions [3]. However, with the development of the supramolecular chemistry of lanthanide complexes, using specially designed ligands, assemblies can be made where the geometry of the metal centre is controlled. This control enables chemists to synthesise assemblies with specific structural properties in order to tune the physical properties.
With a two-photon excitation model in mind, we therefore designed a molecular complex which can maximise the probability of the ETU process. It consists of the connection of two chromium(III) sensitisers around a central erbium(III) acceptor in a linear self-assembled cation (see Figure 95). This system presents several characteristics that can favour the ETU upconversion mechanism such as the high local concentrations of sensitisers, the choice of metals that gives a high efficiency for the Cr-Er energy transfer, and the wrapping of the helical ligand strands around the metal ion that protects them from the solvent.
The structure of such highly paramagnetic slow-relaxing complexes is difficult to assess without the use of X-ray diffraction. NMR and EPR techniques are difficult to use in these systems: NMR suffers from large line widths due to chromium(III) and EPR suffers from complications due to zero-field splitting, TIP effects and large line widths due to erbium(III). But as this compound crystallises with two complexes in the asymmetric unit, the unit-cell is large (volume 47200 Å3). Moreover, disordered solvent molecules occupy channels inside the crystal, and the diffraction data from conventional sources in our laboratory were of poor quality. The higher quality of the data obtained from BM01A, the Swiss-Norwegian beamline, allowed us to unambiguously solve the structure and proved the organisation of the molecule.
The carefully chosen molecular design permitted green emission to be observed upon NIR irradiation of the sample, characteristic of the Er(4S3/2![]() 4I15/2) transition. This first example of upconversion in an isolated molecular complex opens a new playground for synthetic chemists. New molecular designs connecting three Cr ions to the Er ion could for instance lead to unprecedented molecular three-photon upconversion luminescence.
4I15/2) transition. This first example of upconversion in an isolated molecular complex opens a new playground for synthetic chemists. New molecular designs connecting three Cr ions to the Er ion could for instance lead to unprecedented molecular three-photon upconversion luminescence.
Principal publication and authors
L. Aboshyan-Sorgho (a), C. Besnard (b), P. Pattison (c,d), K.R. Kittilstved (e), A. Aebischer (f), J-C. G. Bünzli (f), A. Hauser (e) and C. Piguet (a), Angew. Chem. Int. Ed. 50, 4108–4112 (2011).
(a) Department of Inorganic, Analytical and Applied Chemistry. University of Geneva (Switzerland)
(b) Laboratory of Crystallography, University of Geneva (Switzerland)
(c) Laboratory of Crystallography Ecole Polytechnique Fédérale de Lausanne (Switzerland)
(d) Swiss Norwegian Beamline (SNBL), ESRF
(e) Department of Physical Chemistry University of Geneva (Switzerland)
(f) Laboratory of Lanthanide Supramolecular Chemistry Ecole Polytechnique Fédérale de Lausanne (Switzerland)
References
[1] D.H. Kim and J.U. Kang in Microscopy: Science, Technology, Applications and Education 1, 571-582 (2010).
[2] F. Auzel, Chem. Rev. 104, 139–173 (2004).
[3] C. Reinhard and H.U. Güdel, Inorg. Chem. 41, 1048–1055 (2002).



