- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- X-ray imaging
- The stage of vascular maturation is crucial for tissue damage after microbeam radiation
The stage of vascular maturation is crucial for tissue damage after microbeam radiation
Microbeam radiation has been developed for therapeutic purposes and has been preclinically tested. It is capable of delivering high doses (~150-4000 Gy) of radiation. It involves synchrotron X-rays conditioned as an array of parallel beams of 25-75 µm wide, spaced 100 to 400 µm apart. Compared to broad-beam seamless radiation, currently used in clinical protocols, microbeam radiation has a tissue-sparing effect because of its particular geometry, which produces peak and valley zones of dose deposition [1]. Small vessels have been shown to play an important role in mediating the tissue-sparing effect of microbeam radiation [2].
We have investigated the vascular effects of microbeam radiation relative to the stage of vascular maturation. Moreover, we have evaluated the temporal pathophysiological changes induced by microbeam radiation, compared to those caused by seamless radiation. We chose chick chorio-allantoic membrane (CAM) as the experimental model as it represents an almost pure vascular system, in which maturation of immature capillaries at day 8 (CAM8) of development to mature capillaries at day 12 (CAM12) can be observed [2].
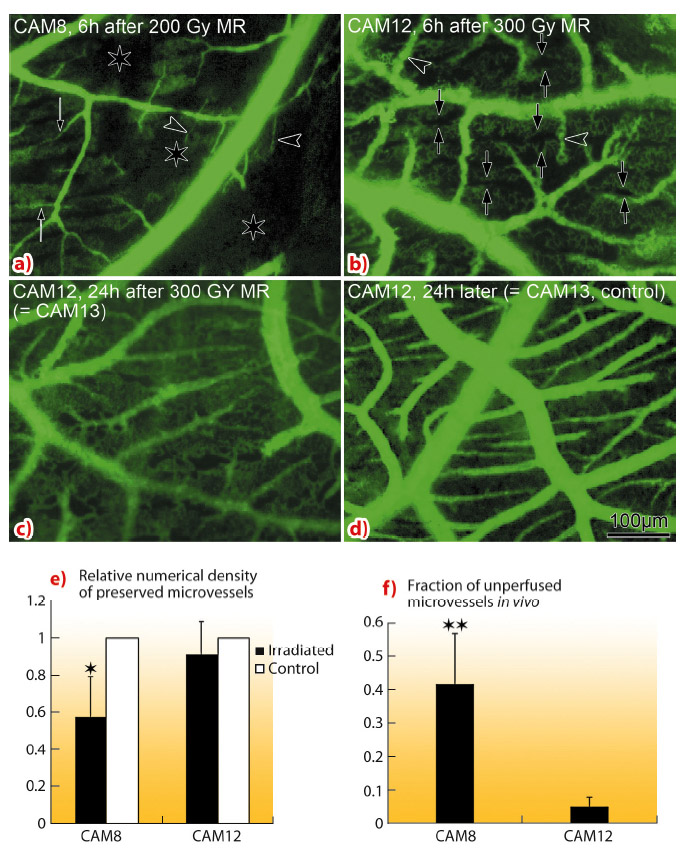
In vivo monitoring (Figure 128) of CAM8 vasculature 6 h after 200 Gy microbeam radiation revealed a near total destruction of the immature capillary plexus. Conversely, a higher dose (300 Gy) barely affected CAM12 mature microvasculature (Figure 128b - d). The quantification revealed that the relative density of preserved microvessels was significantly higher in CAM12 than in CAM8. The fraction of unperfused microvascular areas was significantly lower in CAM12 than in CAM8.
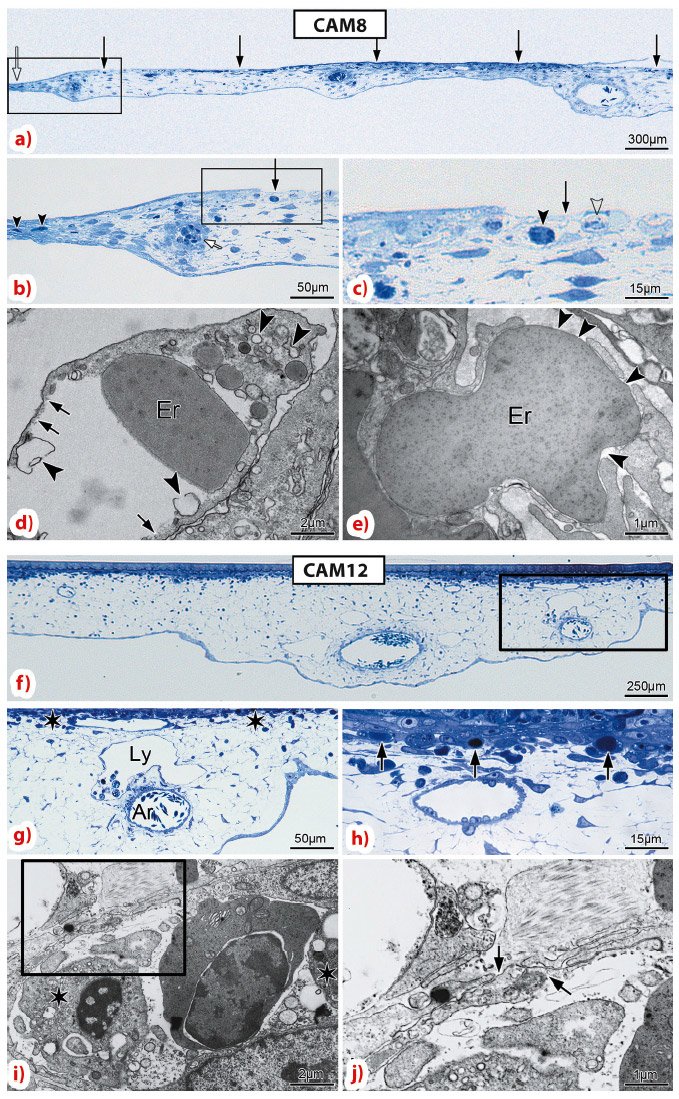
For CAM8, using light and electron microscopy, we observed an increased thickness in the irradiated region 6 h after delivery of 200 Gy microbeam radiation, with a damaged capillary plexus in the beam path (Figure 129). In contrast, for CAM12, the larger blood and lymph vessels were preserved, the capillary plexus remained intact, with only the occasional congested and unperfused capillary.
Dynamic morphological evaluation by light and electron microscopy revealed that the effects of microbeam radiation occur very rapidly: as soon as 15 minutes after 300 Gy, opened interendothelial cell junctions were revealed, which could explain the transient mesenchymal edema with increased CAM thickness. After 30 minutes the capillary plexus displayed a striated metronomic pattern of lesion, alternating damaged and intact zones, corresponding to the beam and the interbeam paths within the array. At 60 minutes, CAM started regaining its normal thickness.
For comparison, a 10 Gy dose of seamless radiation caused growth retardation, while 40 Gy damaged the entire CAM vasculature [2].
Based on our observations, we conclude that the effects of microbeam radiation are mediated by capillary damage, with tissue injury caused by insufficient blood supply. Vascular toxicity and physiological effects of microbeam radiation depend on the stage of capillary maturation and appear in the first 15 to 60 minutes after irradiation. Conversely, the effects of seamless radiation, due to the arrest of cell proliferation, persist for a longer time.
Principal publication and authors
S. Sabatasso (a,c), J.A. Laissue (b), R. Hlushchuk (a,c), W. Graber (c), A. Bravin (d), E. Bräuer-Krisch (d), S. Corde (d,e), H. Blattmann (b,e), G. Gruber (f) and V. Djonov (a,c), Int J Radiat Oncol Biol Phys. 80, 1522-32 (2011).
(a) Institute of Anatomy, University of Fribourg (Switzerland)
(b) Institute of Pathology, University of Bern (Switzerland)
(c) Institute of Anatomy, University of Bern (Switzerland)
(d) ESRF
(e) Radiotherapy Service, Centre Hospitalier Universitaire A. Michallon, Grenoble (France)
(f) Radio Oncology Unit, Clinic Hirslanden, Zürich (Switzerland)
References
[1] E. Bräuer-Krisch, R. Serduc, E.A. Siegbahn, et al., Mutat Res 704, 160-166 (2010).
[2] S. Sabatasso, J.A. Laissue, R. Hlushchuk, et al., Int J Radiat Oncol Biol Phys 80, 1522-32 (2011).