- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Soft condensed matter
- Interaction with synaptic vesicles induces structural changes in a lipid monolayer
Interaction with synaptic vesicles induces structural changes in a lipid monolayer
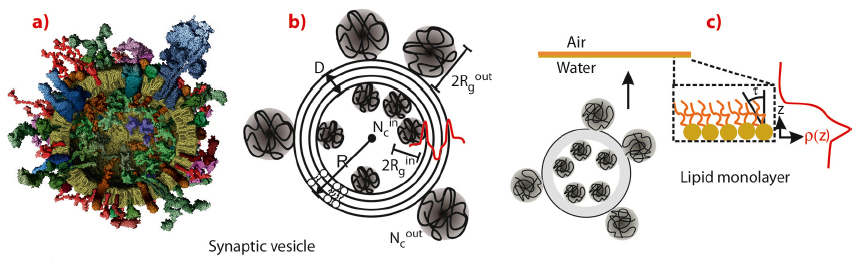
Synaptic vesicles are small, membrane-bound organelles that are found in the synaptic terminal of neurons. The fusion of synaptic vesicles (SVs) with the plasma membrane in neurons is a crucial step in the release of neurotransmitters, which are responsible for carrying signals between nerve cells. After a rise in intracellular Ca2+ during neuronal stimulation, vesicles fuse at discrete sites on the plasma membrane (active zones) releasing their neurotransmitter content, which then passes the signal to neighbouring neurons. While many of the molecular players involved in this fusion process have been identified, a precise description of the structural mechanisms is still lacking. To this end, we have first carried out a small-angle X-ray scattering study on the structure and polydispersity of synaptic vesicles purified from rat brain. In contrast to electron microscopy, the samples can be kept in physiological buffer during the structural characterisation, carried out at beamline ID02. The resulting vesicle diameters, as well as the density and width of the vesicle’s bilayer and the attached native protein layers as quantified by least-square fits to the SAXS curve have confirmed the standard SV model based on biochemical studies [1], see Figure 62.
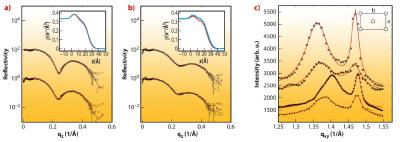
As a next step, we have probed the interaction of SVs with lipid membranes both in the form of bilayers and monolayers, as a simple model of the pre-synaptic membrane. This Ca2+ dependent interaction is thought to be controlled by the vesicular protein synaptotagmin [2] in combination with the “effector” lipid phosphatidylinositol 4,5-bisphosphate (PIP2), a highly negatively charged lipid which resides in the plasma membrane, and acts as a calcium sensor. To test this idea by a structural method, we have carried out X-ray reflectivity and grazing incidence diffraction experiments on a monolayer of dipalmitoyl-sn-glycero-3-phosphatidylcoline (DPPC) with and without addition of PIP2. Using the Langmuir trough setup at beamline ID10B, we observed systematic changes in the lipid film structure at the molecular level after SV injection in the subphase. Along with the pressure increase, a small decrease in the area per lipid as well as the tilt angle (from acyl chain diffraction) in the DPPC film were induced by SV association, along with corresponding changes in the density profile. A collective reorganisation of the lipid film was thus observed, i.e. local binding of a SV leads to a non-local response of acyl chain tilt and film density. Importantly, the relative changes were much more pronounced in the presence of PIP2, see Figure 63. Furthermore, the association was further intensified by a physiologically relevant amount of Ca2+ ions in the subphase of the monolayer, as revealed by the corresponding structural changes and the increase in interfacial pressure [2,3]. The results led us to conclude that the collective structural changes in the lipid induced by only 5% mol. concentration of PIP2 may well modulate vesicle fusion in vivo, and that collective degrees of freedom of the lipid membrane must be taken into account in addition to the specific interactions of fusion proteins.
Principal publication and authors
S.K. Ghosh (a), S. Castorph (a), O. Konovalov (b), R. Jahn (c), M. Holt (c) and T. Salditt (a), New Journal of Physics 12, 105004 (2010); S. Castorph (a), D. Riedel (c), L. Arleth3, M. Sztucki (b), R. Jahn (c), M. Holt (c) and T. Salditt (a), Biophysical Journal 98, 1200-1208 (2010).
(a) Institut für Röntgenphysik, Universität Göttingen (Germany)
(b) ESRF
(c) Max-Planck Institut für biophysikalische Chemie, Göttingen (Germany)
References
[1] S. Takamori et al., Cell 127, 831-846 (2006).
[2] E.R. Chapman, Annu. Rev. Biochem. 77, 615-641 (2008).