- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Soft condensed matter
- Catching a short-lived photoreceptor intermediate with pulsed X-rays
Catching a short-lived photoreceptor intermediate with pulsed X-rays
Protein structural changes in photoreceptors are initiated by the absorption of light. For the photoactive yellow protein (PYP) photoreceptor, structural changes in solutions are much stronger than in the crystalline state [1,2]. We used pump-probe solution X-ray scattering as a structural probe of the photointermediate. By combining the scattering data together with short and long range information from NMR and double electron electron resonance spectroscopy (DEER) data of spin labelled PYP, we have revealed the structural changes.
Extensive investigations have addressed the structural aspects of the signalling mechanism of the PYP photoreceptor. Yet, the details of these structural rearrangements for the signalling state I2’ (pB2) in solution have remained unresolved. In solution, I2’ has the characteristics of a partially unfolded protein, as judged from NMR observations, the loss of secondary structure elements monitored by FTIR and CD spectroscopy, time resolved ORD spectroscopy and altered hydrophobic dye binding and thermodynamic observables. From NMR spectroscopy of I2’ a picture of a partially disordered state has emerged [2].
We used DEER to obtain long-range distance information between selected sites within the protein (including the N-terminal domain) both in the ground and photointermediate I2’ states. Measurement of distance distributions for doubly spin-labelled photoreceptor constructs suggests that the signalling state is well ordered and show that inter-spin-label distances change reversibly by up to 19 Å upon illumination.
 |
|
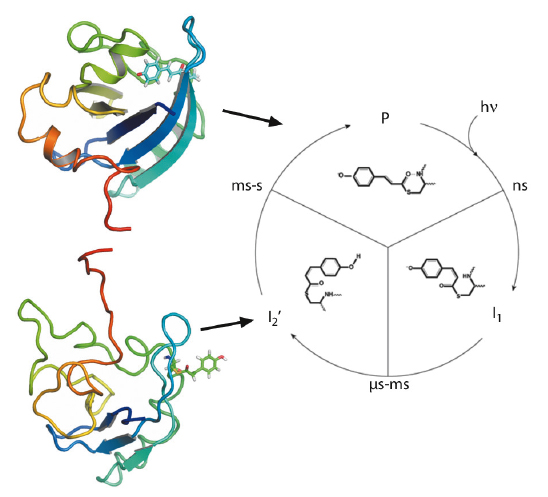
Fig. 66:The photocycle of the photoactive yellow protein is triggered by blue light excitation (λmax = 446 nm), which initiates photoisomerisation of the p-coumaric acid chromophore. Subsequent proton transfer reactions and protein structural changes result in the formation of the signalling state (I2’). In solution, the structural changes are significant and fully reversible. Representative structures for the ground state (P) and the intermediate (I2’) are shown, coloured from N-terminus (red) to C-terminus (blue). |
Next, we performed pump-probe TR-SAXS/WAXS measurements at beamline ID09B to probe the I2’ intermediate. The SAXS/WAXS difference signal for the signalling state, with a 10-ms delay between the optical pump and the X-ray probe, indicates the transient formation of an ordered and rearranged conformation, which has an increased radius of gyration, an increased maximum dimension and a reduced excluded volume. Dynamical annealing calculations using the DEER derived long-range distance restraints in combination with short-range distance information from 1H-15N HSQC perturbation spectroscopy give strong indication for a rearrangement that places part of the N-terminal domain in contact with the exposed chromophore binding cleft while the terminal residues extend away from the core (Figure 66). Time-resolved global structural information from pump-probe TR-SAXS/WAXS data supports this conformation and allows subsequent structural refinement that includes the combined energy terms from DEER, NMR and SAXS/WAXS (Figure 67). The resulting ensemble simultaneously satisfies all restraints and the inclusion of TR-SAXS/WAXS effectively reduces the uncertainty arising from the possible spin label orientations. The observations are essentially compatible with reduced folding of the I2’ state that is widely reported. However, from direct transformations of both the TR-SAXS/WAXS data as well as the DEER data, there is indication that the intermediate is well ordered, comparable to the ground state.
 |
|
Fig. 67: Time-resolved pump-probe SAXS/WAXS of full length wild type PYP. Black: Experimental pump-on minus pump-off difference X-ray scattering data on PYP as a function of q. The X-ray probe pulse is applied 10 ms after the 460 nm pump pulse that probes the I2’ transient population. Blue: Theoretical difference scattering curve obtained using the DEER and NMR derived ensemble for the illuminated form minus the ground state. Red: Theoretical difference scattering curve obtained using the DEER, NMR and SAXS/WAXS derived ensemble for the illuminated form minus the ground state, after dynamical annealing calculations that included all experimental data simultaneously. The bottom panel shows a stereo image of the comparison of the average structures refined with DEER and NMR (blue) and combined DEER, SAXS/WAXS and NMR (red, PDB accession code: 2KX6) restraints simultaneously. |
To the best of our knowledge, here we show the first application that uses simultaneous structure refinement from TR-SAXS/WAXS, DEER and NMR derived restraints. Furthermore, we have applied it to the problem of transient structural change of the PYP photoreceptor.
Principal publication and authors
P.L. Ramachandran (a), J.E. Lovett (b,c), P.J. Carl (d), M. Cammarata (e), J.H. Lee (f), Y.O. Jung (f), H. Ihee (f), C.R. Timmel (b) and J.J. van Thor (g), J. Am. Chem. Soc. 133, 9395-9404 (2011).
(a) Laboratory of Molecular Biophysics, University of Oxford (UK)
(b) Inorganic Chemistry Laboratory, University of Oxford (UK)
(c) EaStCHEM School of Chemistry, University of Edinburgh (UK)
(d) Bruker BioSpin GmbH (Germany)
(e) ESRF
(f) Center for Time-Resolved Diffraction, Department of Chemistry, KAIST (Republic of Korea)
(g) Division of Molecular Biosciences, Imperial College London (UK)
References
[1] H. Ihee, S. Rajagopal, V. Srajer, R. Pahl, S. Anderson, M. Schmidt, F. Schotte, P.A. Anfinrud, M. Wulff and K. Moffat, Proc Natl Acad Sci USA 102, 7145 (2005).
[2] C. Bernard, K. Houben, N.M. Derix, D. Marks, M.A. van der Horst, K.J. Hellingwerf, R. Boelens, R. Kaptein and N.A. van Nuland, Structure 13, 953 (2005).



