- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Soft condensed matter
- Simple ultrasmall peptides self-assemble into fibrous structures found in Alzheimer’s and other degenerative diseases
Simple ultrasmall peptides self-assemble into fibrous structures found in Alzheimer’s and other degenerative diseases
A large number of fatal degenerative diseases including Alzheimer’s exhibit fibrous amyloid aggregates as a common pathological feature. Despite decades of investigations, how pathogenic amyloid structures develop out of naturally occurring proteins remains a mystery. Structural changes of the proteins by misfolding have been identified as one of the most likely causes of amyloid formation. We have rationally designed a novel class of ultrasmall aliphatic peptides of only 3 to 7 amino acids in length that can self-assemble to typical fibrous amyloid structures, see Figure 68 [1].
 |
|
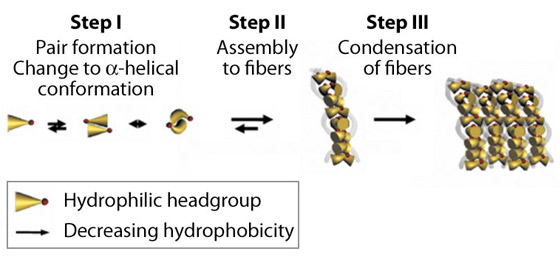
Fig. 68: Hypothetical self-assembly of peptide monomers into supramolecular networks of condensed fibres. Self-assembly is initiated by antiparallel pairing of two peptide monomers by changing to α-helical conformation. Subsequently, peptide pairs assemble to nanostructures and fibres and condense to fibrils resulting in hydrogel formation. |
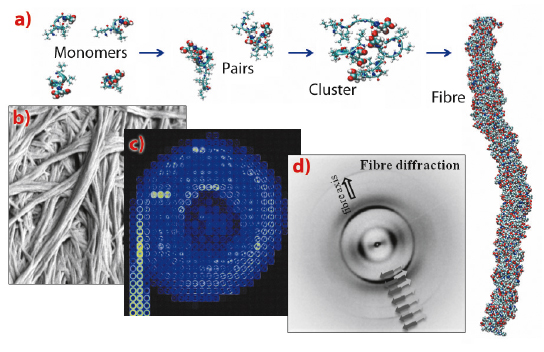
Each of these tri- to heptapeptides contains a water-soluble ‘polar head’ and a water-insoluble ‘tail’ with decreasing hydrophobicity. This specific motif enables the molecules to self-assemble spontaneously in water to form hydrogels—stiff gels held together by stable fibrous aggregates. The honeycomb-like structures of the peptide scaffolds enable them to entrap large amounts of water. We observed a complex stepwise mechanism of aggregation involving at least three different steps. The process of self-assembly to fibres and condensed amyloid aggregates is most likely driven by unexpected α-helical intermediates during the transition to cross-β fibres. Investigations using electron microscopy, spectroscopy and X-ray microdiffraction at the ID13 beamline confirmed these conformational changes (Figure 69). Interestingly, the highly-oriented X-ray fibre diffraction pattern resembled the earlier observed pattern of natural amyloids. It was obtained with a new aggregation technique of peptide solution drops on a superhydrophobic surface, developed in collaboration with the group of E. Di Fabrizio at IIT (Genova). Molecular dynamics simulation of peptide behaviour in water revealed antiparallel pairing of monomers and stable, condensed and coiled fibres.
 |
|
Fig. 69: a) Molecular dynamics simulation of assembly of hexamer-peptide monomers into a fibre. b) The aggregated fibres imaged by scanning electron microscopy. c) Scanning X-ray microdiffraction image of a hydrogel solute drop dried on a superhydrophobic surface. d) Fibre diffraction pattern showing schematically the orientation of the β-strands. |
A particularly challenging aspect of bioengineering is the design of biomimetic materials that closely resemble and reproduce the native three-dimensional architecture and functions occurring in living species. Such a biomimetic material is in great demand for a wide range of biomedical applications such as tissue engineering, in vivo devices for controlled drug delivery or even biochips. Our ultrasmall peptides are designed to self-assemble into a surprisingly wide variety of structures of nano- to supramolecular dimensions [1]. Altering the amino acid sequence of the peptides creates nanostructures, including hollow nanospheres, short, flat fibres, elongated helical fibres and spider web-like structures [2]. Although these hydrogels are soft materials, they possess surprisingly remarkable mechanical strength, far exceeding that of soft tissue in the human body, such as collagen or nucleus pulposus cells (the jelly-like material of the spinal discs). More importantly, the strength can be tuned for applications from injectable therapies to repair and replacement of damaged tissues. To offer an injectable therapy that would render invasive surgery obsolete, the peptides are currently being tested for treatment of degenerative spinal disc disease. Use of these peptide-based gels in cartilage and joint repair as well as wound healing and skin treatment is being explored.
These peptides which closely resemble natural polypeptides can serve as excellent model systems to study the onset and progression of amyloid diseases, its prevention and treatment. An ongoing study investigates ultrasmall peptide therapeutics specifically targeted at the prevention and control of amyloid formation. Furthermore, studying the self-assembly of these peptides, made of simple aliphatic amino acids believed to have existed in the ‘primordial soup’, could provide important clues to the origin of life and the mechanisms underlying evolution.
Principal publication and authors
C.A.E. Hauser (a), R. Deng (a), A. Mishra (a), Y. Loo (a), U. Khoe (a), F. Zhuang (a),D.W. Cheong (b), A. Accardo (c,d), M.B. Sullivan (b), C. Riekel (c), J.Y. Ying (a) and U.A. Hauser (a), Proc. Natl. Acad. Sci. USA 108, 1361-1366 (2011).
(a) Institute of Bioengineering and Nanotechnology (Singapore)
(b) Institute of High Performance Computing (Singapore)
(c) ESRF
(d) Center of BioNanotechnology and Engineering for Medicine (BIOMEMS), University Magna Græcia of Catanzaro (Italy)
(e) Institute of Physics I, University of Cologne (Germany)
References
[1] A. Mishra, Y. Loo, R. Deng, Y.J. Chuah, H.T. Heeb, J.Y. Ying and C.A.E. Hauser, Nano Today 6, 232-239 (2011).
[2] A. Lakshmanan and C.A.E. Hauser, Int. J. Mol. Sci. 12, 5736-5746 (2011).



