- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Electronic structure and magnetism
- X-ray absorption spectroscopy reveals natural organic matter as major sorbent for arsenic in peat
X-ray absorption spectroscopy reveals natural organic matter as major sorbent for arsenic in peat
Approximately 6% of the global ice-free land area is covered by wetlands, which play an important role in the storage, transformation, and mobilisation of trace elements like arsenic originating from natural and anthropogenic sources. In wetland soils and peaty sediments, natural organic matter (NOM) is thought to control the mobility and bioavailability of As by promoting its release from As-bearing metal-oxyhydroxides or, conversely, by triggering the formation of As-sequestering sulphides under strongly reducing conditions. Numerous wetland systems as well as peaty sediments, however, show enrichments of As not obviously related to the presence of As-bearing minerals, suggesting a direct immobilisation of As by NOM. Although several modes of As-NOM interactions, for example, ternary As complex formation or covalent binding of As by functional groups of NOM, have been proposed in the literature, spectroscopic evidence was missing so far. Here, we analysed the solid-phase speciation of As and Fe in a naturally As-enriched (<1,800 mg As/kg), slightly acidic minerotrophic (groundwater-fed) peatland located in southern Switzerland by X-ray absorption spectroscopy (XAS). Our main objectives were to (i) provide spectroscopic evidence for As binding to NOM, (ii) identify the governing binding mechanism(s), and (iii) quantify the extent of As binding to NOM. Peat samples collected at the field site were immediately shock-frozen in liquid N2 to preserve the speciation of As, transported on dry ice to the laboratory, freeze-dried, and processed in an anoxic glove box. Based on mineralogy and elemental composition, selected peat samples were prepared for XAS analyses by sieving the material to a particle size <500 μm. The samples were then placed in Plexiglas sample holders, sealed with Kapton® tape, and kept anoxic until the final XAS measurements. Arsenic K-edge (11,867 eV) XAS spectra were collected at beamline BM29 in fluorescence-yield mode at approximately 80 K using a N2 Oxford cryostream. The XAS spectra were analysed by means of principal component analysis and target transformation testing followed by linear combination fitting as well as shell fitting. Linear combination fit analyses showed that close to the peat surface, As was present as As-NOM complexes (AsIII), realgar-type sulphide minerals (As4S4, AsII), and As sorbed to Fe(III)-oxyhydroxides (AsIII/V).
 |
|
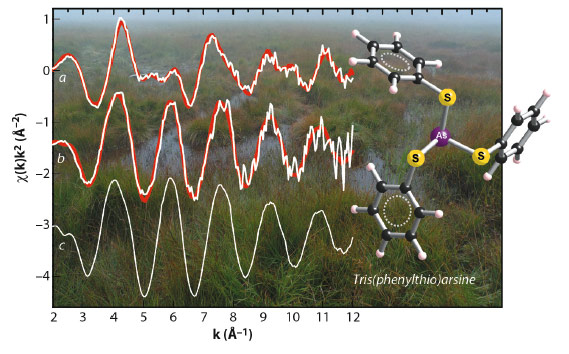
Fig. 100: Arsenic K-edge EXAFS spectra of two representative peat samples (a, b) and the organic As reference compound tris(phenylthio)arsine (c). The peat samples were collected from a depth of 0-10 cm (a) and 190-200 cm (b). Experimental data is shown as white lines and linear combination fits of the peat samples as red lines. |
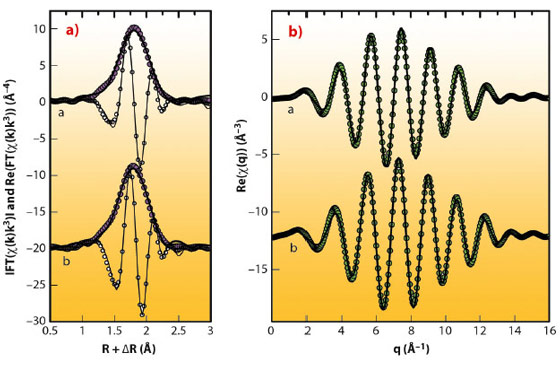
In contrast, samples of deep peat layers were entirely dominated by As-NOM complexes (Figure 100), despite the presence of potentially As-sequestering Fe(III)-oxyhydroxides and pyrite (FeS2). Shell-fit analysis of the extended X-ray absorption fine structure (EXAFS) spectra of these samples revealed that AsIII was coordinated to 2-3 S atoms with an average interatomic As-S distance of 2.26 ± 0.01 Å (Figure 101). This distance is identical to interatomic As-S distances of thioarsine model compounds (Figure 101) and thus implies that trivalent As in the peat was bound to 2-3 sulphydryl groups of NOM. These findings demonstrate for the first time that in anoxic NOM- and S-rich environments, NOM can be a quantitatively important sorbent for As, capable of successfully rivalling mineral phases known to have a high affinity towards As – a result which highlights the need to account for NOM in geochemical speciation models used to assess the toxicity and mobility of As in anoxic NOM-rich environments. Overall, our findings suggest that the binding of As to sulphydryl groups of NOM represents the major As-NOM interaction mode in anoxic NOM-rich environments subject to moderately sulphate-reducing conditions. The spectroscopic analyses conducted at the ESRF have thus greatly improved our understanding of the biogeochemical As cycle and are key to interpreting As-NOM associations observed in other European peatlands, as well as in peats and peaty sediments of Southeast Asia.
Principal publication and authors
P. Langner, C. Mikutta and R. Kretzschmar, Nat. Geosci. 5, 66-73 (2012).
Soil Chemistry Group, Institute of Biogeochemistry and Pollutant Dynamics, ETH Zurich (Switzerland)