- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structure of materials
- Design of zeolite by inverse sigma transformation: Cut and paste zeolites
Design of zeolite by inverse sigma transformation: Cut and paste zeolites
Zeolites are crystalline microporous aluminosilicates which consist of corner-sharing TO4 tetrahedra. They are workhorses in industry for adsorption, separation and catalysis. Heteroelements are often inserted to enhance desired properties such as catalytic activity and selectivity. The introduction of germanium however results in stability problems.
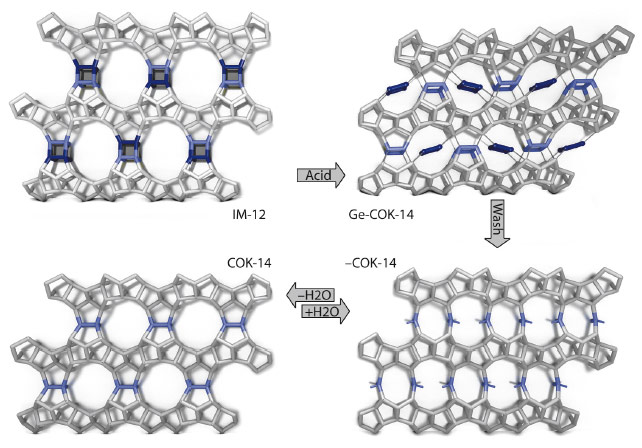
Germanosilicate IM-12 zeolite (UTL topology) consists of dense silicate layers connected by double-four rings (D4Rs), the preferential location of germanium (Figure 138). The systematic instability of the UTL zeolite can be used to harvest building units that can be rearranged to transform UTL zeolite into a new and stable zeolite. Acid leaching removes the germanium from the UTL framework while preserving the dense silicate layers and reconnects these layers to form new zeolite topologies –COK-14 or COK-14. –COK-14 is a new all-silica interrupted framework topology with a two-dimensional channel system and interconnecting 8-, 10- and 12-membered rings (Figure 138). Upon drying in the absence of water, silanol condensation takes place and COK-14 is formed (Figure 138). The transformation from UTL zeolite to COK-14 involves the systematic removal of one layer of T-atoms and as such is the first experimentally observed inverse sigma transformation.
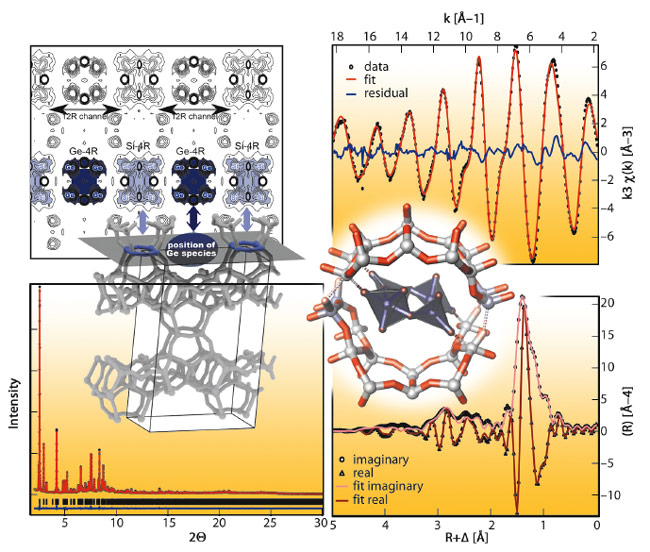
To verify if a true inverse sigma transformation occurred, an intermediate state was captured named Ge-COK-14. This sample was synthesised by reducing transformation time. 29Si-NMR and 1H-NMR indicated this sample contained Q3 silicon environments, associated with silanol groups, while still having Si-O-Ge bonds. Approximately 50 wt% of the original germanium content was still present in Ge-COK-14. High-resolution powder X-ray diffraction data and X-ray absorption spectra for Ge-COK-were collected at room temperature at beamline BM01B (the Swiss Norwegian Beamline SNBL) in collaboration with beamline BM26 (DUBBLE) (Figure 139). Indexing of the powder pattern of Ge-COK-14 resulted in a unit cell of a = 24.43 Å, b = 13.90 Å, c = 12.28 Å and a monoclinic angle of 108.97° compared to a = 24.64 Å, b = 13.92 Å, c = 12.26 Å and β = 109.20° for –COK-14 and a = 29.00 Å, b = 13.98 Å, c = 12.45 Å and β = 104.91° for the parent UTL zeolite. This indicated that Ge-COK-14 already had the interlayer spacing of –COK-14 but still contained germanium. Ge K-edge EXAFS spectra revealed the local environment and coordination of Ge in Ge-COK-14 (Figure 139). Each Ge atom had two Ge neighbours at 3.2 Å. A combination of HRXRD and Ge-K-edge EXAFS measurements allowed the structure of Ge-COK-14 to be unravelled (Figure 138, Figure 139). In this sample, germanium was present as a germanate four-ring in the position that would later become the 12-membered ring of COK-14. Ge-K-edge EXAFS measurements of the parent UTL zeolite revealed this germanate four-ring was already present (Figure 138).
Acid leaching removes the germanate four-ring from the UTL zeolite and shifts it into the channels of Ge-COK-14. Prolonging the transformation and washing leads to full elimination of germanium and results in the new zeolite frameworks –COK-14 and COK-14 (Figure 138). The COK-14 zeolite family is a valuable addition to the all-silica large-pore zeolite types. More new zeolites with attractive pore architectures may be obtained by this inverse sigma transformation approach.
Principal publication and authors
E. Verheyen (a), L. Joos (b), K. Van Havenbergh (c), E. Breynaert (a), N. Kasian (a,d), E. Gobechiya (a), K. Houthoofd (a), C. Martineau (e), M. Hinterstein (f), F. Taulelle (e), V. Van Speybroeck (b), M. Waroquier (b), S. Bals (c), G. Van Tendeloo (c), C.E.A. Kirschhock (a) and J.A. Martens (a), Nat. Mater. 11, 1059–1064 (2012).
(a) Center for Surface Chemistry and Catalysis, KU Leuven (Belgium)
(b) Center for Molecular Modeling, Ghent University (Belgium)
(c) Electron Microscopy for Materials Science, University of Antwerp (Belgium)
(d) L.V. Pisarzhevsky Institute of Physical Chemistry, National Academy of Sciences of Ukraine, Kyiv (Ukraine)
(e) Tectospin, Institut Lavoisier, UMR 8180 Université de Versailles (France)
(f) Institut für Werkstoffwissenschaft, Technische Universität Dresden (Germany)