- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structural biology
- An unexpected role in coffee beans for a protein normally associated with lignin biosynthesis
An unexpected role in coffee beans for a protein normally associated with lignin biosynthesis
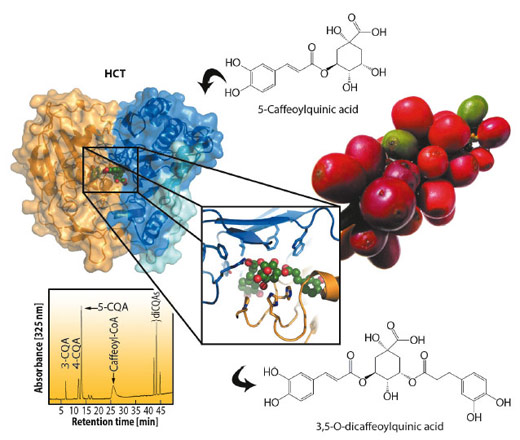
Functional food research is a new scientific discipline that focuses on using food for both sustenance and as a delivery vehicle for bioactive molecules. Coffee is an example of such a functional food as it can be used both as a drink and to deliver the well-known bioactive molecule caffeine. However, coffee also contains other less well-known bioactive molecules, including the chlorogenic acids (CGAs). The CGAs are a group of soluble secondary metabolite esters that have been implicated in biotic and abiotic stress responses in plants, while the related shikimate esters are key intermediates for lignin biosynthesis. One or more CGAs are found in important food species such as potatoes, tomatoes, apples and pears, with a particularly high level of 5-caffeoylquinic (5-CQA) and 3,5-O-dicaffeoylquinic acid (3,5-diCQA) found in coffee beans (Figure 22). In addition to their role as secondary metabolites in plants, there is growing evidence that the CGAs can act as dietary antioxidants and that their consumption can have beneficial effects for human health. So, enjoying your morning cup of coffee not only invigorates your mind but could also be beneficial for your overall health.
The CGA and shikimate esters are synthesised via the phenylpropanoid pathway and the last step is catalysed by the hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl-transferases (HCT/HQT). HCT and HQT can reversibly shuttle hydroxycinnamoyl units between their CoA- and shikimate/quinate-esterified forms, but have a preference for shikimate and quinate respectively. HCT and HQT belong to the plant specific BAHD aceyltransferase family. Despite their generally low sequence identity, these enzymes possess a sequence conserved catalytic HXXXD motif and can be further phylogenetically distributed into eight distinct clades. While some structural information was available for several BAHD family members, no structural information was available for the clade containing HCT/HQT. Members of this large family are involved in the biosynthesis of a large array of secondary metabolites, including a number of important therapeutic drugs. In an effort to understand the remarkable substrate diversity of the BAHD superfamily, we performed structural, biochemical and mutagenesis studies of HCT and HQT from Coffea canephora (robusta) in collaboration with groups at Nestlé R&D in Tours, France and Washington University in St. Louis Missouri, USA.
Native crystals of HCT appeared only once in initial crystal trials and access to the microfocus capability of beamline ID23-2 were crucial to successful data collection from very thin plates. We then designed a more proteolytically stable variant of HCT that readily crystallised.
We subsequently solved several structures of HCT from different conditions using data collected at beamline ID14-4. A comparison of these different structures revealed a remarkably plasticity in and around the active site. Unfortunately, we were never able to obtain a structure of HQT or a substrate bound HCT structure. Nevertheless, using ligand docking algorithms, structural and sequence comparisons, as well as the molecular modelling of HQT we were able to identify putative residues important in substrate recognition (Figure 22). Several of these were mutated and characterised biochemically to validate the docking results. In particular, Leu400 and Phe402, of which the side chains were predicted to be important for shikimate binding in HCT, were mutated to the Thr and Tyr residues found in HQT, and resulted in a shift of the substrate preference to quinate, as expected. We also unexpectedly showed for the first time that, in vitro, HCT is capable of synthesising the 3,5-O-diCQA. This novel activity is consistent with our ligand docking experiments and helps explain why green coffee beans contain such a large quantity of diCQAs. During our mutagenesis studies we also identified a new HCT mutant, His-153-Asn, designed to mimic the catalytic loop of HQT, which resulted in a significantly increased 3,5-diCQA production (Figure 22).
These results provide the first structural characterisation of a hydroxycinnamoyl transferase from the BAHD family and in conjunction with our docking, biochemical and mutagenesis studies reveal the molecular level determinants for the substrate specificity of this important family of proteins. This work has potential applications in altering the level of the beneficial CGAs and diCGAs compounds in other food plant species or in the generation of novel target compounds. As HCT is central to the biosynthesis of complex lignins, our results could also be used to modulate the substrate specificity of HCT and form the basis for new research directions in the area of biofuel production from non-food plant biomass.
Principal publication and authors
L.A. Lallemand (a), C. Zubieta (a), S.G. Lee (b), Y. Wang (c), S. Acajjaoui (a), J. Timmins (a), S. McSweeney (a), J.M. Jez (b), J.G. McCarthy (d) and A.A. McCarthy (e), Plant Physiology 160, 249–260 (2012).
(a) ESRF
(b) Department of Biology, Washington University, St. Louis (USA)
(c) Donald Danforth Plant Science Center, St. Louis (USA) (d) Nestlé Research and Development, Notre-Dame d’Oé, Tours (France)
(e) UVHCI, UJF-EMBL-CNRS, Unité Mixte Internationale 3265 and EMBL, Grenoble (France)