- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Electronic structure and magnetism
- Investigation of electronic structure and magnetism of bistable molecules with X-ray absorption
Investigation of electronic structure and magnetism of bistable molecules with X-ray absorption
Molecular material science, developed in the last century for electronics, photonics and sensors applications, is currently moving towards the scaling down of devices to the single molecule level. For this, a single molecule, or a series of them, has to be integrated into the hybrid nanostructure of the device. Moving from the bulk to the single molecule requires analytical tools with appropriate sensitivity. Synchrotron light has great potential for this through the use of X-ray absorption-based spectroscopy (XAS) techniques. Here we summarise the results of the experiments carried out at beamline ID08 on two classes of molecular systems, a molecular nanomagnet [1] and a valence tautomeric system [2], characterised respectively by a molecular magnetic memory effect and light and temperature induced switching of the magnetic and electronic properties.
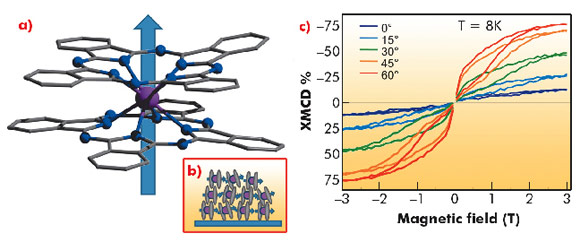
One of the simplest and most frequently studied molecular nanomagnets is obtained by assembling a single terbium (III) ion with two phthalocyanine molecules (TbPc2) in the double decker neutral structure shown in Figure 74. This molecule, at cryogenic temperatures, features a magnetic hysteresis - hence the name single molecule magnet - and the origin of this behaviour is related to the magnetic anisotropy of the single molecule. TbPc2 can be evaporated and assembled in a quasi-crystalline structure depending on the thickness of the film, as we have recently demonstrated [3]. The measurement of the amplitude of the dichroic contribution at the Tb M4,5 edge (shown in Figure 74) as a function of the applied magnetic field yields the magnetisation curve of this molecular system, clearly evidencing the presence of slow dynamics in the magnetisation that depends on the orientation of the film with respect to the external field.
This effect originates from the molecular arrangement: TbPc2 assembles in a standing configuration with the easy-axis of the magnetisation parallel to the surface, as confirmed using linear polarised light in an X-ray natural linear dichroism (XNLD) experiment [3]. Different behaviour is observed for a monolayer deposited on gold, where the molecules assemble in the lying-down configuration, with the easy-axis of the magnetisation perpendicular to the surface. In this case the magnetisation dynamics are faster.
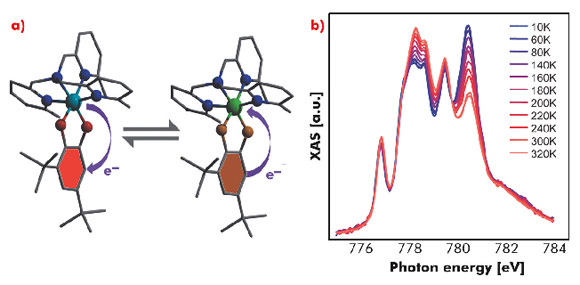
Another intriguing class of molecules studied at ID08 belongs to the Co-dioxolene (Co-Diox) family. These systems display temperature-, pressure- or light-driven intramolecular electron transfer between the metallic core and the surrounding dioxolene ligand, resulting in the possibility of switching their electronic configuration with an external stimulus. The states involved in this valence tautomerisation process – low spin-CoIII-catecholate, diamagnetic and dominant at low temperature, and high spin-CoII – semiquinonate, paramagnetic and favoured at high temperatures – can be directly followed in an XAS experiment. It was possible to follow the tautomerisation of the Co-Diox system as a function of temperature by measuring the fine structure of the Co L3 absorption edge (Figure 75). The light induced switching of the molecules to high spin-CoII at low temperature could be triggered by IR laser light. An analogous process has been found to arise from X-ray irradiation, suggesting the existence of an additional mechanism for the photo-induction [4].
 |
|
Fig. 75: a) Structure of the Co-Diox system and representation of the valence tautormerism. b) Co L3 XAS spectra of the Co-Diox system as a function of temperature. |
Both experiments represent key steps towards the use of X-rays as fundamental tools to study technologically important and innovative molecular based materials for use in future high-tech devices.
Authors
M. Mannini (a), G. Poneti (a), L. Margheriti (a), Ph. Sainctavit (b), A. Dei (a) and R. Sessoli (a).
(a) Department of Chemistry “Ugo Schiff” & INSTM research unit, University of Florence (Italy)
(b) Institut de Minéralogie et de Physique des Milieux Condensés, CNRS, Université Pierre et Marie Curie, Paris (France)
References
[1] D. Gatteschi, R. Sessoli and J. Villain Molecular nanomagnets, Oxford University Press, Oxford, UK (2006).
[2] A. Dei, D. Gatteschi, C. Sangregorio and L. Sorace, Acc. Chem. Res. 37, 827-835 (2004).
[3] G. Poneti, M. Mannini, L. Sorace, P. Sainctavit, M.-A. Arrio, E. Otero, J.C. Cezar and A. Dei, Angew. Chem. Int. Ed. 49, 1954-1957 (2010).
[4] D. L. Margheriti, D. Chiappe, M. Mannini, P.-E. Car, P. Sainctavit, M.-A. Arrio, F.B. de Mongeot, J.C. Cezar, F.M. Piras, A. Magnani, E. Otero, A. Caneschi and R. Sessoli, Adv. Mater. 22, 5488-5493 (2010).