- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Electronic structure and magnetism
- Absence of Ce3+ sites in chemically active colloidal ceria nanoparticles
Absence of Ce3+ sites in chemically active colloidal ceria nanoparticles
Materials based on ceria (CeO2) have a capacity to store and release oxygen in their intrinsic n-type fluorite structure. This provides them with unique redox properties that are widely used and offers potential for promising applications in the areas of catalysis, fuel cells, and biomedicine. Developing tailored CeO2 materials for industrial applications involves a clear understanding of the chemical activity of CeO2. This ultimately depends on the electronic structure, which can be controlled at the nanoscale by the morphology.
Reducing the size of CeO2 to the nanoscale enhances the chemical activity. The generally advocated mechanism states that particle miniaturisation results in an increase in the surface to bulk ratio and thus produces a significantly non-stoichiometric CeO2-δ owing to surface oxygen vacancies. Electrons left behind by O-vacancy formation are believed to reduce Ce4+ to Ce3+ ions, resulting in a higher level of catalytically active Ce3+ ions at the surface that are responsible for the increased chemical activity of nanoceria when compared to bulk ceria. This ionic description of the catalytic activity is commonly supported by experiments performed under conditions that are very different from the environment of the working catalyst and may even change the chemical state of ceria (e.g. high vacuum). The electronic structure of ceria nanoparticles critically depends on the reaction environment, thus experiments need to be performed under in situ / operando conditions to fully reproduce the environment under which activity is observed.
We monitored the electronic structure of ceria nanoparticles using high energy resolution fluorescence-detected hard X-ray absorption spectroscopy (HERFD-XAS) at beamline ID26. This technique allows spectral features to be observed beyond the limitation of the L3 edge lifetime broadening [1]. In the case of rare earth systems, HERFD-XAS reveals the fine structure of the chemically active 4f orbitals and thus provides a direct, in situ probe of the Ce chemical state (Figure 21).
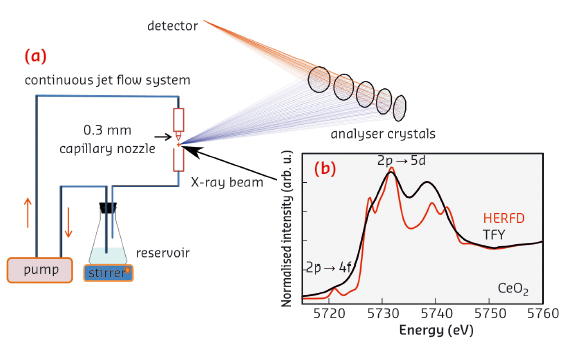 |
|
Fig. 21: a) Experimental setup for HERFD-XAS of colloidal nanoparticles. The suspension is circulated and the spectra are recorded on a free liquid jet. b) Comparison between conventional total fluorescence yield (TFY) with HERFD Ce L3-edge spectra in bulk CeO2. The electronic transitions are assigned to the spectral features. |
A simple ionic picture has been shown to be insufficient to describe inner-shell spectroscopic data of ceria and a multi-configuration approach is necessary. The electronic structure of Ce in bulk CeO2 with formal valence state of IV can be described as a mixture of f0 and f1L contributions (where L denotes a hole in a ligand orbital) thus considering orbital mixing and sharing of electron density between Ce and O. Such formalism implies that the system remains in a singlet spin state (S = 0) independent of the f0 and f1L mixing ratio because the Ce f electron is spin paired with a ligand electron. The resulting spectral shape is very different from a system with two distinct f0 (Ce4+) and f1 (Ce3+) sites where the spectrum presents the sum of the two sites. Oxygen deficient CeO2-δ may be described by an increased f1L contribution in presence of strong orbital mixing and an unchanged singlet spin state. Such a description, however, contradicts the generally accepted view of a Ce3+ site with a local, unpaired spin moment in chemically active nanoceria [2].
We tested the catalytic activity of the ceria NPs by monitoring the decomposition of hydrogen peroxide (H2O2). This reaction mimics the activity of catalase, a common enzyme in organisms that balances the level of reactive oxygen species and thus protects cells. The 3 nm nanoparticles show rapid ( < 1 minute) and significant changes in the HERFD-XAS (Figure 22) that are accompanied by an increase in the Ce-O inter-atomic distance. The pre-edge peak remains as one strong resonance, leading us to conclude that Ce is in a singlet state over the whole course of reaction. The changing intensity ratio between the two strong 5d absorption peaks can be rationalised as a change in the f0 and f1L contributions to the ground state electronic structure.
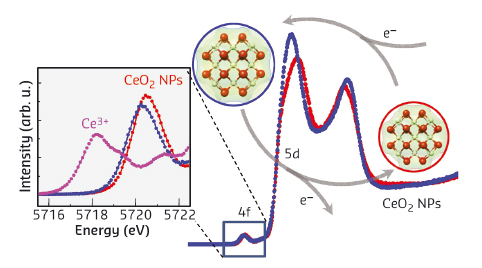 |
|
Fig. 22: Spectral changes in ceria nanoparticles (NPs) with average size of 3 nm after synthesis in ethanol (blue) and in the presence of H2O2 (red). The inset shows a magnification of the 2p → 4f transitions and a comparison to Ce3+ in Ce(NO3)3. |
Thus, the picture of a delocalised electron distribution emerges where the entire NP participates in the reaction as opposed to single Ce3+ sites. The challenge now is to find a quantum chemical theoretical description of the electron structure that accounts for the particle morphology and is able to model X-ray absorption data.
Principal publication and authors
J.-D. Cafun (a), K.O. Kvashnina (a), E. Casals (b,c) , V.F. Puntes (b,c) and P. Glatzel (a), ACS Nano 7, 10726–10732 (2013).
(a) ESRF
(b) Institut Català de Nanotecnologia (ICN), Bellatera (Spain)
(c) Universitat Autònoma de Barcelona (UAB), Bellaterra (Spain)
References
[1] K. Hämäläinen, D.P. Siddons, J.B. Hastings and L.E. Berman, Phys. Rev. Lett. 67, 2850-2853 (1991).
[2] F. Esch, S. Fabris, L. Zhou, T. Montini, C. Africh, P. Fornasiero, G. Comelli and R. Rosei, Science 309, 752-755 (2005).



