- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Dynamics and extreme conditions
- Rattling modes in thermoelectric sodium cobaltate
Rattling modes in thermoelectric sodium cobaltate
The design of better thermoelectric materials would enable the reduction of energy consumption in cars by converting waste heat in exhausts into useful electrical power, as well as cooling hot spots on computer chips using solid state refrigerators. However, the need for both high electrical conductivity and low thermal conductivity creates a design conflict. Here we show how rattling modes suppress the thermal conductivity in the electrically conducting thermoelectric oxide, sodium cobaltate.
The so-called square superstructure of Na0.8CoO2 shown in Figure 35 comprises arrays of tri-vacancy clusters [1]. We have performed first-principles density-functional calculations for the lattice dynamics of this large-period superstructure on the UK’s national supercomputer facility HECToR using the CASTEP code, and the results are presented as a colour contour diagram in Figure 36. The calculated spectrum differs markedly from that of the stoichiometric compound at energies below E ~ 20 meV. In particular, there is an additional narrow mode at E ~ 13 meV in the superstructure that is completely absent for NaCoO2.
 |
|
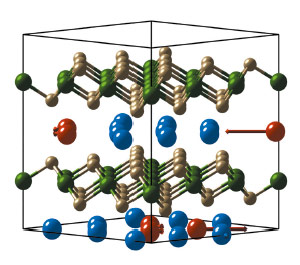
Fig. 35: The supercell of Na0.8CoO2 showing the Einstein-like rattling mode at E ~ 13 meV comprising mainly displacements of the (red) Na 2b ions inside tri-vacancy clusters. Na 2d ions are blue, Co is green and O is gold. |
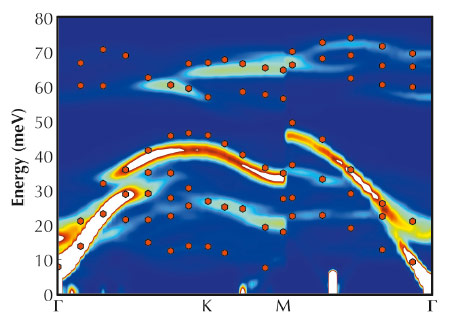
Inelastic X-ray scattering (IXS) measurements were performed on a single crystal of Na0.8CoO2 of a few hundred micrometres in size with the square superstructure using the ID28 spectrometer. Scans of variable energy transfer were performed at a number of settings of momentum transfer Q along high-symmetry directions, and the fitted peak positions are shown in Figure 36 as red hexagons. The agreement between the ab initio calculations and the IXS experimental data is remarkable.
 |
|
Fig. 36: The phonon dispersion of Na0.8CoO2 in the square superstructure. First-principles density-functional calculations for the square array of tri-vacancy clusters are shown as a colour contour diagram on a hot scale (blue, low intensity; white, high intensity). The IXS data are shown as red hexagons. The superstructure is essential to capture the scattering at lower energies below E ~ 20 meV. |
We observe the approximately flat mode at E ~ 13 meV. It is possible to trace this mode back to the zone centre, and the relative amplitudes of the ionic displacements are shown in Figure 35. The blue spheres are Na 2d sites, and these are fully occupied in the stoichiometric compound. The red spheres are on the inequivalent Na 2b sites and, because they are associated with clusters of three vacancies, they are expected to be more loosely bound. The atomic displacement pattern is characterised by a predominant in-plane amplitude of vibration of the Na 2b sites with negligible contribution from other sites. Thus we find that the Na 2b sites inside tri-vacancy clusters sandwiched between CoO2 layers are rattler sites, and the mode at E ~ 13 meV is a rattling mode.
The measured energy line widths are sharp, indicating relatively long phonon lifetimes. There is, therefore, no evidence for the level of phonon scattering required by the phonon-glass electron-crystal model. The lattice contribution to the thermal conductivity calculated using the measured phonon dispersion and lifetimes was found to be in quantitative agreement with experiment. The rattling modes at low energy contribute towards the specific heat, but have small propagation velocities, and they suppress the thermal conductivity by a factor of six compared with vacancy-free NaCoO2.
In conclusion, we are able to unambiguously identify an Einstein-like rattling mode at low energy and, as a result, to quantitatively account for the suppression of thermal conductivity for this class of materials. Our approach using IXS can readily be extended to other candidate materials, since we only require tiny crystals, and the results will guide the design of the next generation of thermoelectric materials.
Principal publication and authors
D.J. Voneshen (a), K. Refson (b), E. Borissenko (c), M. Krisch (c), A. Bosak (c), A. Piovano (d), E. Cemal (a,d), M. Enderle (d), M.J. Gutmann (e), M. Hoesch (f), M. Roger (g), L. Gannon (h), A.T. Boothroyd (h), S. Uthayakumar (a), D.G. Porter (a) and J.P. Goff (a), Nature Materials 12, 1028 – 1032 (2013).
(a) Royal Holloway, University of London (UK)
(b) Science and Technology Facilities Council (UK)
(c) ESRF
(d) Institut Laue-Langevin (France)
(e) ISIS Facility (UK)
(f) Diamond Light Source (UK)
(g) CEA Saclay (France)
(h) Oxford University (UK)
References
[1] M. Roger, D.J.P. Morris, D.A.Tennant, M.J. Gutmann, J.P. Goff, J.-U. Hoffmann, R. Feyerherm, E. Dudzik, D. Prabhakaran, A.T. Boothroyd, N. Shannon, B. Lake and P.P. Deen, Nature 445, 631-634 (2007).



