- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Dynamics and extreme conditions
- Effect of nanostructuring on the electronic properties of cathode materials
Effect of nanostructuring on the electronic properties of cathode materials
Rechargeable batteries have applications in many fields including portable electronic consumer devices, electric vehicles, and large-scale electricity storage in smart or intelligent grids. LiCoO2 has been extensively used as cathode material in Li-ion batteries because of its best performance in terms of high specific energy density and excellent life cycle [1,2]. Nevertheless, a relatively slow diffusion of Li-ions in the bulk system limits its efficiency. Nanostructured electrodes of LiCoO2 have been proposed as a solution to improve the cathode performance since the nanoparticles have unique physical and chemical properties with respect to the bulk materials [3,4]. Moreover, there appears to be a critical particle size below which the use of smaller nanoparticles is no longer beneficial [4]. In particular, reduction of the crystallite size to less than 15 nm drastically decreases the capacity of LiCoO2.
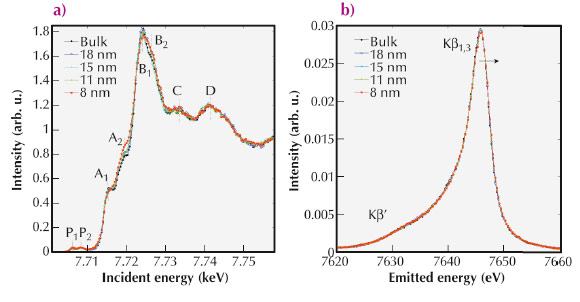
We have investigated the effect of nanostructuring on the electronic properties of LiCoO2 by high-resolution X-ray absorption and X-ray emission spectroscopy. The measurements reported in Figure 41 were performed at the former beamline ID16 on a series of LiCoO2 nanoparticles of different sizes. Panel (a) shows the Co K-edge absorption spectra. Thanks to the wavelength dispersive crystal spectrometer, we were able to achieve the high resolution necessary to resolve the low energy absorption components P1 and P2 (panel (a)). While the pre-peak P1 is due to 1s → eg local quadrupole transitions with a dipolar contribution due to Co 4p-3d mixture arising from the distorted octahedral environment of Co, feature P2 corresponds to 1s → 4p-3d non-local dipole transitions related to oxygen mediated metal-metal interactions. Panel (b) reports the Co Kβ emission spectra (3p → 1s). The absence of a pronounced feature on the lower energy side (Kβ’) is an indication of a low-spin state for the full set of samples. Subtle variations of the emission spectra correspond to the variation of the Co local magnetic moment and of the Co oxidation state as a function of the nanoparticle size.
 |
|
Fig. 41: (a) Normalised partial Kb fluorescence yield absorption spectra of LiCoO2 across the Co K-edge. Spectra relative to different nanoparticle sizes from the bulk to 8 nm are reported. (b) Co Kβ X-ray emission spectra of LiCoO2 for different nanoparticle sizes. |
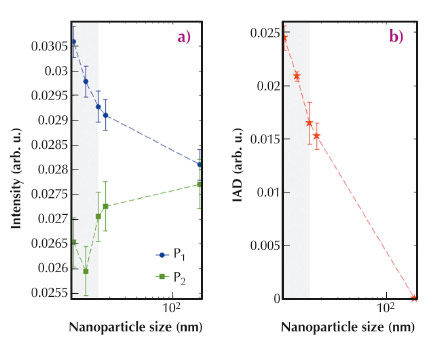
By analysing the high resolution Co K-edge absorption spectra and the Co Kβ emission spectra we extract quantitative information on the intersite/intrasite hybridisation and on the Co oxidation state and local magnetic moment. We found that reduction of the particle size leads to a change in the electronic structure similar to the delithiation effect [5]. Below a nanoparticle size of 15 nm, the intersite (intrasite) 4p-3d hybridisation shows an abrupt decrease (increase) that is correlated to the variation of the local Co magnetic moment (Figure 42).
 |
|
Fig. 42: (a) Evolution of pre-peak features P1 and P2 in the Co K-edge XAS spectra as a function of nanoparticle size. While P1 increases, P2 tends to decrease with decreasing nanoparticle size, showing an abrupt drop below 15 nm. (b) The integrated absolute value of the difference spectra (IAD) of the Kβ emission spectra with respect to a non magnetic reference are proportional to the local 3d spin magnetic moment, µ. IAD with respect to the bulk is shown as a function of nanoparticle size. |
Our results provide important information on the understanding of the reduced electrochemical efficiency for LiCoO2 nanoparticle with a critical value of 15 nm.
Principal publication and authors
L. Simonelli (a,b), N.L. Saini (c), M. Moretti-Sala (a), M. Okube (d), I. Honma (e), T. Mizokawa (f), and G. Monaco (a,g), Applied Physics Letters 103, 083111 (2013).
(a) ESRF
(b) ALBA Synchrotron Light Facility, Barcelona (Spain)
(c) Dipartimento di Fisica, Università di Roma “La Sapienza” (Italy)
(d) National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, (Japan)
(e) Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai (Japan)
(f) Department of Physics and Department of Complexity Science and Engineering, University of Tokyo (Japan)
(g) Dipartimento di Fisica, Università di Trento (Italy)
References
[1] J.-M. Tarascon and M. Armand, Nature 414, 359 (2001).
[2] P. He, H. Yu, D. Li and H. Zhou, J. Mater. Chem. 22, 3680 (2012).
[3] A.S. Arico, P. Bruce, J.-M. Tarascon and W.V. Schalkwijk, Nature Mater. 4, 366 (2005).
[4] M. Okubo, E. Hosono, J. Kim, M. Enomoto, N. Kojima, T. Kudo, H. Zhou, and I. Honma, J. Am. Chem. Soc. 129, 7444 (2007); M. Okubo, J. Kim, T. Kudo, H. Zhou and I. Honma, J. Phys. Chem. C 113, 15337 (2009).
[5] L. Maugeri, A. Iadecola, B. Joseph, L. Simonelli, L. Olivi, M. Okubo, I. Honma, H. Wadati, T. Mizokawa and N.L. Saini, J. Phys.: Condens. Matter 24, 335305 (2012).



