- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Soft condensed matter
- Ideal polyethylene nanocrystals
Ideal polyethylene nanocrystals
Polymers such as polyethylene rarely form single crystals and, upon cooling, an entangled polymer melt usually solidifies into crystalline lamellae consisting of regularly folded chains sandwiched between extended amorphous regions. This peculiar nanostructure primarily controls the mechanical properties and aesthetic features of polymers. Thanks to recent advances in catalytic polymerisation, polyethylene-nanocrystals consisting of single lamellae can be formed in aqueous dispersion [1]. Annealing of such crystals leads to a thickening of the crystalline lamella that can be studied with high precision [2]. However, the role of short chain branches and entanglements remained unclear in all studies done so far. A possible approach that overcomes these problems is a compartmentalisation of the polymerisation and then a perfect ordered deposition of the nascent chain on a nanocrystal growth front. We have realised this scheme by means of a new catalyst that produces virtually perfect polyethylene chains that are free of branches.
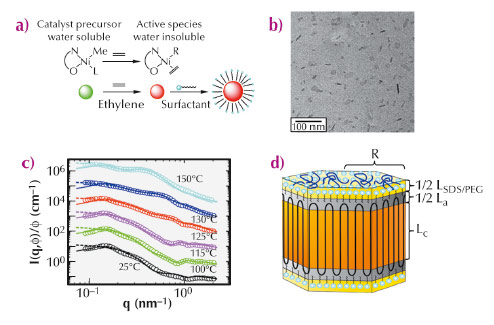
Figure 72a summarises the main steps of the catalytic polymerisation and the chemical structure of the catalyst. Upon activation, the catalyst becomes lipophilic and polyethylene chains, which are formed immediately, pack into surfactant-stabilised particles. These polyethylene chains were found to be linear (Mn = 4.2 105 g mol-1 and Mw / Mn = 1.4) and virtually devoid of any branches. Figure 72b displays a micrograph of the dispersion taken by cryogenic transmission electron microscopy (cryo-TEM), indicating well-defined nanocrystals with relatively narrow size distribution and a faceted shape. To further elucidate the semicrystalline nanostructure, we have performed a comprehensive structural analysis of the polyethylene-nanoparticles by small-angle X-ray scattering (SAXS) at beamline ID02. Typical SAXS profiles together with model fits are shown in Figure 72c. Figure 72d illustrates the platelet model used for the data analysis consisting of a crystalline lamella that is enclosed by two amorphous layers.
 |
|
Fig. 72: Catalytic polymerisation of ethylene in aqueous suspension and the formation of poly(ethylene) nanocrystals. (a) Polymerisation of ethylene in water. (b) Cryo-TEM micrograph of the polyethylene-nanocrystals in aqueous dispersion. (c) Normalised SAXS intensities from aqueous suspensions of polyethylene nanocrystals annealed at indicated temperatures. For clarity, successive curves are multiplied by a factor 10. The dashed lines represent the results of the modelling of the SAXS data by a dispersion of non-interacting particles and the solid lines by interacting particles, respectively. (d) Scheme of the platelet-like structure of the nanoparticles used for the SAXS analysis, indicating the thick crystalline part, thin layer of the amorphous turns and outer stabilising surfactant layer. |
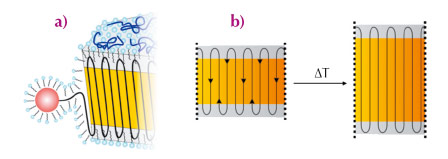
SAXS investigation of the annealed samples revealed that the thickness of the crystalline layer increases when annealed at 100°C and 115°C while the thickness of the amorphous layer remains constant. Thus, the crystallinity increases markedly and finally reaches about 88%. This finding supports the idea of an ideal polyethylene-nanocrystal: the absence of branches allows the polyethylene-chains to diffuse through the crystal without melting or major distortion. The amorphous layers hence act like the wheels of a pulley as depicted schematically in Figure 73. This in turn shows clearly that the amorphous regions are virtually devoid of entanglements. The growing chains emanating from the catalytic centre are laid down on the facet of the polyethylene-nanocrystals in an orderly fashion, that is, without the formation of entanglements. The high degree of crystallinity was further confirmed by the wide angle X-ray scattering analysis.
 |
|
Fig. 73: Proposed mechanism for the formation of ideal polyethylene-nanocrystals. (a) The polyethylene-chain emanating from the catalytic centre is stabilised in the aqueous phase by the adsorbed surfactant SDS. The growing chain is then laid down on a given facet of the polyethylene-nanocrystal in an orderly fashion without entanglements or other defects. (b) Mechanism of thermal annealing in an ideal polyethylene-nanocrystal: the amorphous layers covering both ends of platelets act like the wheels of a pulley, just changing the direction of the chains. |
In summary, this study could open up a new type of crystal engineering that leads to ideal polymer nanocrystals with improved processing capabilities as a result of their non-entangled nature, especially for ultra-high molecular weights, and robust mechanical performance.
Principal publication and authors
A. Osichow (a), C. Rabe (b), K. Vogtt (b), T. Narayanan (c), L. Harnau (d), M. Drechsler (e), M. Ballauff (b) and S. Mecking (a), JACS 135, 11645-11650 (2013).
(a) Chemical Materials Science, Department of Chemistry, University of Konstanz (Germany)
(b) Helmholtz-Zentrum Berlin for Materials and Energy and Department of Physics, Humboldt University, Berlin (Germany)
(c) ESRF
(d) Max Planck Institute for Intelligent Systems and Institute for Theoretical Physics IV, University of Stuttgart (Germany)
(e) Bayreuth Institute of Macromolecular Research, University of Bayreuth (Germany)
References
[1] C.H.M. Weber, A. Chiche, G. Krausch, S. Rosenfeldt, M. Ballauff, L. Harnau, I. Göttker-Schnetmann, Q. Tong and S. Mecking, S. Nano Lett. 7, 2024-2029 (2007).
[2] C.N. Rochette, S. Rosenfeldt, K. Henzler, F. Polzer, M. Ballauff, Q. Tong, S. Mecking, M. Drechsler, T. Narayanan and L. Harnau, Macromolecules 44, 4845-4851 (2011).



