- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- X-ray imaging
- Synchrotron X-ray fluorescence shows reduced neuronal copper in vulnerable brain regions in Parkinson’s disease
Synchrotron X-ray fluorescence shows reduced neuronal copper in vulnerable brain regions in Parkinson’s disease
Neurodegenerative cascades in Parkinson’s disease (PD) involve protein aggregation and oxidative stress. Although the triggers for these events are unknown, changes in biometals have long been suspected to play a role [1]. Copper is an important biometal in the brain, as exemplified by Menkes’ and Wilson’s diseases, which are serious neurological disorders of Cu dyshomeostasis. Furthermore, the complexing of Cu with the PD-associated protein α-synuclein increases aggregation and toxicity of this protein, possibly via stimulation of free radical production [2]. But Cu is also an essential cofactor in a range of cuproproteins, including the key protective cellular antioxidant superoxide dismutase 1 (SOD1).
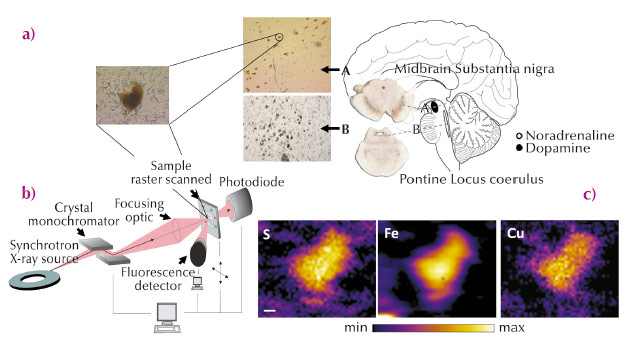
A major obstacle for the investigation of metals in neurodegenerative disease, however, has been the absence of suitable methods to study intracellular changes in the location and levels of metals and the broader regional distribution of metals in the postmortem human brain. Synchrotron radiation X-ray fluorescence microscopy (SRXFM) is ideal for highly sensitive, high resolution quantitative investigations of metals at the cellular level in the brain [3], as exemplified in Figure 23. We used SRXFM at beamline ID22 (now closed and transferred to ID16) to investigate metals in individual surviving pigmented neurons within the degenerating substantia nigra pars compacta (SN) and locus coeruleus (LC), as well as in the non-degenerating occipital cortex, in the brains of cases with PD, incidental Lewy body’s disease (ILBD) or Alzheimer’s disease (AD), and compared with controls. Results from these single cell SRXFM studies were confirmed using microparticle induced X-ray emission (µPIXE) at the AIFIRA facility, CNBG, France, to quantify regional tissue metal levels. Copper (Cu) levels from non-degenerating tissues of the occipital cortex were similar in all cases. In contrast, Cu was significantly reduced within individual pigmented neurons of the degenerating SN and LC in PD and in ILBD, a disorder suggested as representing a preclinical stage of PD [4].
 |
|
Fig. 23: (a) Anatomical location of the human brain nuclei studied. Typical section from the locus coeruleus (LC), substantia nigra pars compacta (SN) and a magnified view of typical neuromelanin (NM)-containing neurons from healthy human brain analysed by synchrotron radiation X-ray fluorescence microscopy (SRXFM) (b) Experimental set-up for SRXFM. (c) shows representative SRXRF maps for selected elements in a single intact NM-containing neuron of the SN shown in (a). Sulfur (S), iron (Fe) and copper (Cu). Scale bar 5 µm. |
Quantitative analysis of fixed sections by SRXFM revealed a decrease up to 45% in neuromelanin (NM)-associated Cu in the SN from PD and ILBD cases with respect to both age-matched controls and AD cases. Our finding of decreased NM-associated Cu levels in the PD SN was confirmed in fresh frozen tissue sections. µPIXE analysis of NM-containing neurons confirmed a decrease in Cu-associated NM in PD and ILBD compared with controls. Similarly, Cu decrease was observed for NM from LC neurons in PD compared with controls, while no differences were seen in the grey matter of the occipital cortex. This data confirms a specific reduction in Cu within remaining NM-pigmented neurons in PD and shows that this deficit occurs in cases with ILBD and limited cell loss, suggesting an early deficit that precedes cell death and clinical symptoms. As Cu levels were not altered in the degenerating LC in AD, it is unlikely that the reduction in Cu observed in these regions in PD results simply from neurodegeneration per se. Furthermore, as Cu levels were unchanged in regions that do not degenerate in PD, it appears that this early decrease is specific to regions vulnerable to neuronal cell loss in this disorder.
We therefore examined the distribution and cellular localisation of Cu transport proteins, and activity of the protective antioxidant, and cuproprotein, superoxide dismutase 1 (SOD1), in post-mortem human brains with a pathological diagnosis of PD, compared with controls, using inductively coupled plasma-mass spectrometry, western blot, and immunofluorescence. We identified a marked reduction in neuronal Cu transport protein 1 (Ctr1) immunoreactivity in the SN in PD. Further, in the PD SN, neuron-associated Ctr1 levels were significantly correlated with Cu levels. In these same PD cases, in brain regions displaying α-synuclein pathology, SOD1 specific activity was altered to reflect the pattern of cell loss. The reductions in cellular Ctr1 and Cu would compromise the ability of these neurons to defend themselves against high levels of oxidative stress, consistent with the severity of cell loss observed in these PD-vulnerable brain regions. Further investigation of cupropathology in PD may identify novel targets for the development of protective therapies for this disorder.
Principal publication and authors
K.M. Davies (a), S. Bohic (b), A. Carmona (c), R. Ortega (c), V. Cottama (a), D.J. Hare (d), J.P.M. Finberg (d), S. Reyes (a), G.M. Halliday (a), J.F.B. Mercer (f) and K.L. Double (a), Neurobiology of Aging 35, 858-866 (2014).
(a) Sydney Medical School, University of Sydney (Australia)
(b) Inserm U836, Grenoble (France)
(c) CENBG - CNRS, Gradignan (France)
(d) Melbourne University (Australia)
(e) Faculty of Medicine, Technion, Haifa (Israel)
(f) Deakin University, Melbourne (Australia)
References
[1] S. Bolognin, L. Messori and P. Zatta, Neuromolecular Med. 11, 223-238 (2009).
[2] X. Wang, D. Moualla, J.A. Wright and D.R. Brown, J Neurochem. 113, 704-714 (2010).
[3] S. Bohic, K. Murphy, W. Paulus et al., Anal Chem. 80, 9557-9566 (2008).
[4] D.W. Dickson, H. Braak, J.E. Duda et al., Lancet Neurol. 8:1150-7 (2009).



