- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Electronic structure and magnetism
- Rate capability enhancement by Ru doping in high voltage lithium-ion battery cathode material LiNi0.5Mn1.5O4
Rate capability enhancement by Ru doping in high voltage lithium-ion battery cathode material LiNi0.5Mn1.5O4
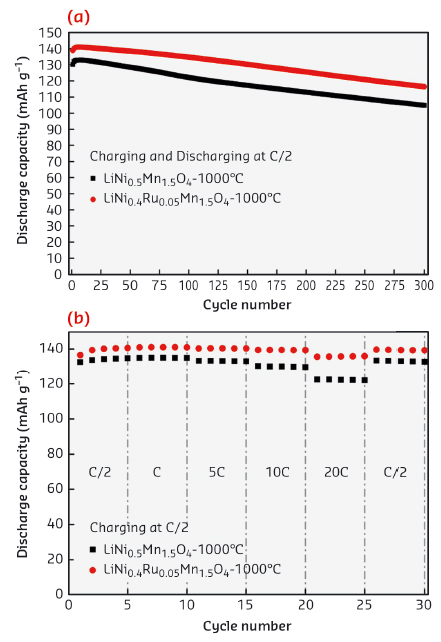
Lithium-ion batteries are the most common energy storage systems commercialised so far for applications in electric vehicles or hybrid electric vehicles. The application of Li-ion batteries in electromobility demands high volumetric as well as gravimetric energy and power densities which are factors often determined by the positive electrode or cathode materials [1]. Nowadays, most of the commercial lithium-ion batteries employ layered Li(Ni,Co,Mn)O2 and spinel-type LiMn2O4 (working at ~4 V) as cathode and graphite as anode. However, the spinel type cathode materials possess high cycling stability in comparison to the layered counterpart. Spinels have a general formula AB2O4, where the oxygen atoms occupy 32e site (Fd3m space group) and form a face-centred cubic close packing. The A cations occupy tetrahedral 8a site, whereas the B cations are located in octahedral 16d site. The unoccupied octahedral 16c site together with the 8a tetrahedral site forms a three dimensional pathway for the lithium-ion diffusion in these materials. Ni-doped Mn spinel with the composition LiNi0.5Mn1.5O4 (LNMO) is considered as highly promising cathode material due to its large reversible capacity obtained at a high operating voltage of around 4.7 V where the reversible Ni2+ ↔ Ni4+ redox reactions take place [2]. In this work, we have proved that doping of the LNMO with Ru further increases the rate capability and cycling stability. The Ru doped spinel (LNRMO) with the composition LiNi0.4Ru0.05Mn1.5O4 was prepared by citric acid-assisted sol-gel method with heat treatment at 1000°C for the final compound. The material has an initial capacity of ~139 mAh g-1 and capacity retention of 84% after 300 cycles at C/2 charging-discharging rate between 3.5-5.0 V vs. Li/Li+. The achievable discharge capacity at 20C for a charging rate of C/2 is ~136 mAh g-1 (~98% of the capacity delivered at C/2). These results are summarised in Figure 27.
 |
|
Fig. 27: a) Cycle number vs. discharge capacity plots at C/2 charge-discharge rate. b) Cycle number vs. discharge capacity plots at C/2 charge rate and varied discharge rates for Ru-doped and un-doped LNMO samples cycled between 3.5-5.0 V at room temperature. |
 |
|
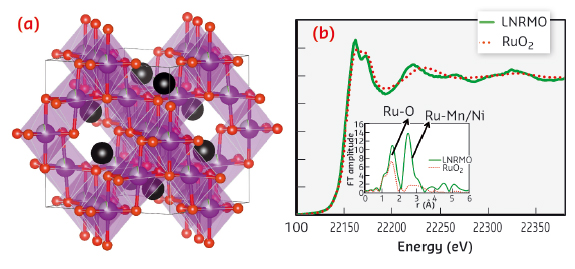
Fig. 28: a) Atomic cluster of LiNi0.4Ru0.05Mn1.5O4 spinel (Red: oxygen; purple: Ni, Mn and Ru, black: Li). b) The Ru K-edge XANES of LNRMO compared to the reference RuO2, reveals that the oxidation state of Ru is +4. The Fourier transform of the EXAFS clearly shows a strong second peak confirming Ru bonding to the transition metal (Mn/ Ni). |
The Ru doping inside the spinel material was confirmed by XAS studies carried out at beamline BM23. From the XANES analysis of the Ru K edge data, Ru was found to be in +4 oxidation state. The EXAFS analysis shows that Ru has a nearest coordination bond distance of 2.02 Å and 2.94 Å with oxygen and transition metal Ni or Mn. This conclusively proves that the Ru exists inside the spinel lattice (Figure 28).
Principal publication and authors
N. Kiziltas-Yavuz (a), A. Bhaskar (a), D. Dixon (a), M. Yavuz (a,b), K. Nikolowski (a,c), L. Lu (d), R. Eichel (e,f) and H. Ehrenberg (a,b), J. Power Sources 267, 533-541 (2014).
(a) Karlsruhe Institute of Technology (KIT), Institute for Applied Materials - Energy Storage Systems (IAM-ESS), Eggenstein-Leopoldshafen (Germany)
(b) Helmholtz Institute Ulm (HIU) Electrochemical Energy Storage, Karlsruhe (Germany)
(c) Fraunhofer Institute for Ceramic Technologies and Systems IKTS, Dresden (Germany)
(d) National University of Singapore, Dept. of Mechanical Engineering (Singapore)
(e) Forschungszentrum Jülich, Institut für Energie- und Klimaforschung (IEK-9) (Germany)
(f) RWTH Aachen University, Institut für Physikalische Chemie (Germany)
References
[1] C.M. Julien and A. Mauger, Ionics. 19, 951-988 (2013).
[2] A. Bhaskar, D. Mikhailova, N. Kiziltas-Yavuz, K. Nikolowski, S. Oswald, N.N. Bramnik and H. Ehrenberg, Progress in Solid State Chemistry 1, 1-21 (2014).



