- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Electronic structure and magnetism
- Local fivefold symmetry in liquid and undercooled Ni
Local fivefold symmetry in liquid and undercooled Ni
Perfect crystal structures can contain pyramids, cubes, or hexagons, but not pentagons. The five-fold symmetry of a pentagon is indeed impossible to replicate over and over in space to make a conventional crystal. Most solid metals actually form closely packed cubic crystals, but in the 1950s, calculations showed that metals such as copper, gold, silver, and lead might form liquids with local five-fold symmetry, in the form of icosahedral structures. The presence of such close-packed fivefold configurations, favoured by energetic considerations, was considered as a possible explanation for the peculiar undercooling properties of liquid metals. Crystal nucleation is hampered by the positive liquid-crystal interfacial energy, resulting in the possibility of bringing a liquid sample into a metastable (undercooled) molten state below the melting temperature. However, five-fold ordering in metals is not entirely unprecedented. Quasicrystals are metal alloys that have a peculiar semi-crystalline five-fold symmetry that never quite repeats itself. The determination of the local structure in liquid matter is quite a challenging task. Most techniques (X-ray and neutron diffraction) can only find the relative positions of two atoms at a time, which makes it difficult to pin down the five-fold symmetry prediction. On the other hand, X-ray absorption spectroscopy (XAS) is a technique particularly sensitive to short range ordering and able to determine also three-body distributions. In this work, we used the ESRF XAS beamline [1] (now BM23) to perform XAS measurements combined with complementary techniques, such as fixed energy X-ray absorption temperature scans and X-ray diffraction. Reaching extremely high temperatures, we have investigated the short-range structure in solid and liquid Ni, following a previous study on liquid Cu [2] and other metals. Samples were prepared from a submicrometric Ni and alumina (Al2O3) powder mixtures with a 1:20 mass ratio that were suspended in alcohol, filtered, and pressed into 100-μm-thick, 13-mm-diameter pellets. The furnace consisted of a 130-mm-diameter cylindrical Pyrex glass vessel with a suitable window for X-rays. The pellets were placed inside the crucible of the furnace and the heat treatments were performed under high-vacuum conditions (P = 10-5 mbar). Temperature was monitored using a high-temperature pyrometer. X-ray absorption at fixed energy as a function of temperature is shown in Figure 29.
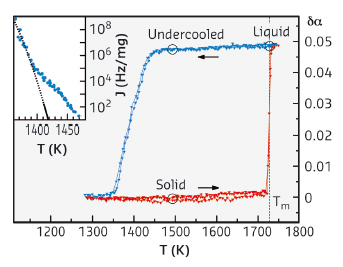 |
|
Fig. 29: X-ray absorption temperature scans of the Ni sample. The change in the absorption coefficient at E = 8.338 keV is plotted with respect to the solid Ni absorption level during repeated thermal cycles. Inset: temperature dependence of the Ni nucleation rate from the cooling scan. |
Upon cooling, the absorption remains stable about 300 K below the melting point, showing the deep undercooling properties of small metal particles. The relatively broader decrease in absorption is associated with the progressive nucleation of the broad mass droplet distribution in the sample. Analysis of this part of the scan using previous methods allows us to extract the Ni nucleation rate in a relatively wide temperature range (Figure 29, inset). High quality EXAFS spectra have been collected in the solid phase at 1493 K, in the stable liquid phase at 1733 K and in the undercooled liquid phase at 1493 K. The extracted signals are compared in Figure 30.
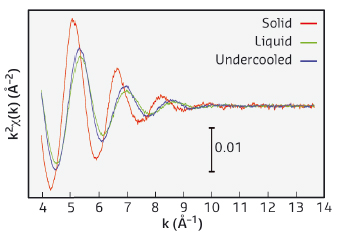 |
|
Fig. 30: EXAFS extracted signals for different phases of Ni. |
Using a sophisticated data-analysis technique, known as Reverse Monte Carlo (RMC), coupled with GNXAS multiple-scattering calculations, it has been possible to create a model structure for the liquid reproducing our experimental data with some constraints. Moreover, ab initio computer molecular-dynamics (MD) simulations provided further insights to the determination of the local structure. From both RMC and MD configurations we were able to calculate different physical quantities, such as bond angle distribution functions. Two different approaches have been used to analyse the local geometry: the common-neighbour analysis (CNA) and the calculation of spherical harmonic invariants, giving consistent results. The fractions of fivefold nearly icosahedral configurations found were in the range 14-18% for Ni in the liquid undercooled phase and 11-14% for liquid Ni just above the melting temperature. The emerging picture for the liquid structure is that of a mixture of nearly icosahedral structures embedded in a disordered network mainly composed of fragments of highly distorted icosahedra, structures reminiscent of the crystalline phase and other configurations.
Principal publication and authors
A. Di Cicco (a), F. Iesari (a), S. De Panfilis (b), M. Celino (c), S. Giusepponi (c) and A. Filipponi (d), Phys. Rev. B 89, 060102(R) (2014).
(a) Università di Camerino (Italy)
(b) IIT, Roma (Italy)
(c) ENEA Casaccia (Italy)
(d) Università dell’Aquila (Italy)
References
[1] A. Filipponi, M. Borowski, D.T. Bowron, S. Ansell, S. De Panfilis, A. Di Cicco and J.P. Itié, Rev. Sci. Instr. 71, 2422 (2000).
[2] A. Di Cicco, A. Trapananti, S. Faggioni and A. Filipponi, Phys. Rev. Lett. 91, 135505 (2003).



