- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- X-ray Imaging
- The in vivo radiosensitising effects of nanoparticle-based MRI contrast agents
The in vivo radiosensitising effects of nanoparticle-based MRI contrast agents
Radiotherapy is commonly used in cancer treatment, either alone or in combination with surgery and/or chemotherapy, since X-rays induce lethal alterations of the tumour. However, the sparing of the surrounding healthy tissue remains a challenge because the similarities in the atomic composition of the tumour and the surrounding tissues do not favour a preferential absorption of the therapeutic beam. For this reason, the use of radiosensitisers composed of heavy elements (high atomic number, Z), combined with a preferential accumulation in the tumour tissues, has been proposed in order to increase the ratio between the dose deposited in the tumour and the dose deposited in the surrounding healthy tissues [1]. In this context, gadolinium plays an important role because of its high relaxivity value and because of its relatively high atomic number value (Z = 64), highlighting its potential for MRI guided radiation therapy [2].
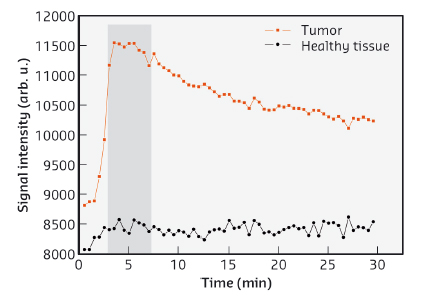
Studies using gold nanoparticles coated with gadolinium chelates have already been carried out and the design of the particles optimised to allow both MRI monitoring (owing to the gadolinium chelates) and radiosensitisation (due to the gold core, Z = 79). The delay between the administration of the nanoparticles and the radiotherapy session was determined from the data collected by MRI (Figure 61) prior to irradiation. Although better results are expected by refining the biodistribution of the nanoparticles inside the tumour, the lifespan of the rats bearing glioma was enhanced by combining microbeam radiation therapy (MRT) and nanoparticle injection resulting in lifespans extended by 473%, which compares favourably to the 222% obtained when MRT was used without nanoparticles.
 |
|
Fig. 61: Temporal evolution of the MRI signal in tumour (red squares) and in an equivalent surface in normal tissue in the left hemisphere (black circles) after intravenous injection of gadolinium chelate coated gold nanoparticles. The grey area represents the time interval during which the difference of signal intensities is the highest. |
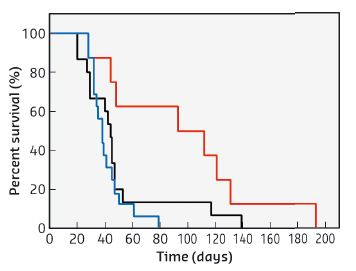
The potential for image guided radiotherapy of gadolinium based nanoparticles was compared to routinely used gadolinium chelates, by using a glioma model implanted in the rat brain. Viewed by MRI imaging, a high and relatively constant concentration was conserved by the tumour while the nanoparticles were rapidly cleared from healthy tissues. This is far better than for gadolinium chelates which are rapidly eliminated from the tumour. Another advantage of this protocol is that the same injection can be used for both MRI and radiotherapy. When radiotherapy was carried out at beamline ID17 using identical experimental conditions, the combination of gadolinium nanoparticles and radiotherapy allowed the median survival time of rats bearing glioma to be doubled when compared to the use of only gadolinium chelates. These results are shown in Figure 62.
 |
|
Fig. 62: Survival curves of 9LGS bearing rats treated by MRT only (black curve, n = 15 rats), treated by MRT 20 minutes after injection of DOTAREM® (blue curve, n = 16 rats), and of AGuIX nanoparticles (red curve, n = 8 rats). The irradiation was performed 10 days after tumour implantation. |
To conclude, two kinds of gadolinium based nanoparticles were used both in MRI and radiotherapy in order to develop the concept of image guided radiotherapy, i.e. in order to use the same contrast media on images as a dose media during radiotherapy. The long time of residence of the nanoparticles (when compared to chelates) within the tumour allows a radiotherapy session to be carried out when the ratio between the gadolinium content of the tumour versus the surrounding healthy tissues is optimal. This time delay can be assessed by MRI prior to the radiotherapy. Finally, both gadolinium and gold/gadolinium based nanoparticles were proven to enhance the lifespan of rats bearing glioma when compared to radiotherapy alone.
Principal publication and authors
I. Miladi (a), C. Alric (a), S. Dufort (b), P. Mowat (a), A. Dutour (c) , C. Mandon (a), G. Laurent (d), E. Bräuer-Krisch (e), N. Herath (f), J.L. Coll (g), M. Dutreix (f), F. Lux (a), R. Bazzi (d), C. Billotey (a), M. Janier (a), P. Perriat (h), G. Le Duc (e), S. Roux (d) and O. Tillement (a). Small 10, 1116-1124 (2014).
G. Le Duc (e), S. Roux (d), A. Paruta Tuarez (a), S. Dufort (b), E. Brauer-Krisch (e), A. Marais (a), C. Truillet (a), L. Sancey (a), P. Perriat (h), F. Lux (a) and O. Tillement (a), Cancer Nanotechnology 5:4 (2014).
(a) Institut Lumière Matière, Université de Lyon (France)
(b)Nano-H S.A.S, Saint Quentin-Fallavier (France)
(c) Centre Léon Bérard, Lyon (France)
(d) Institut UTINAM, Université de Franche-Comté, Besançon (France)
(e) ESRF
(f) Institut Curie, Orsay (France)
(g) Institut Albert Bonniot, Grenoble (France)
(h) MATEIS, INSA, Lyon (France)
References
[1] J.F. Hainfeld, D.N. Slatkin and H.M. Smilowitz, Phys. Med. Biol. 49, 309-315 (2004).
[2] D. Francis, G.M. Richards, A. Forouzannia, M.P. Mehta and D. Khuntia. Expert. Opin. Pharmacother. 10, 2171-2180 (2009).



