- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Structure of materials
- Microbeam diffraction gives new insights into the working of lithium-ion battery electrodes
Microbeam diffraction gives new insights into the working of lithium-ion battery electrodes
This work aimed at revealing the local structural changes in Li-ion electrodes during charge and discharge, which are decisive for battery performance as they limit fast charging and play a crucial role in the restricted cycle life of Li-ion batteries.
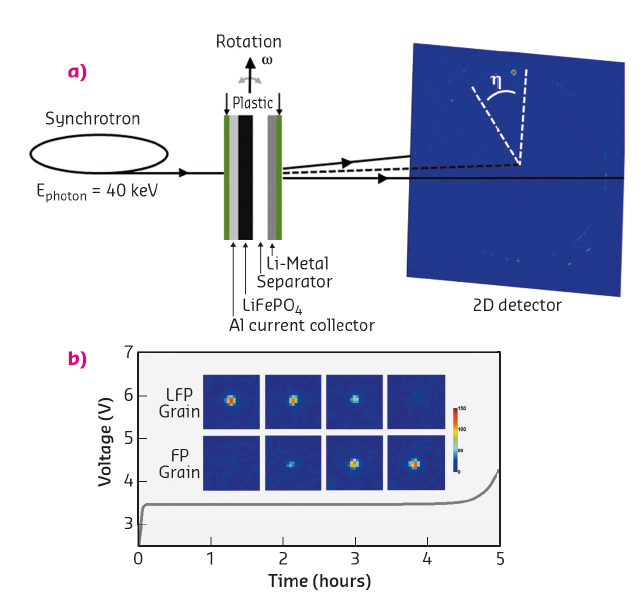
The overall phase transformation behaviour of Li-ion battery electrode materials is well known. However, even for well-studied electrode materials such as LiFePO4, it is unknown how the transformation proceeds within individual grains and how it is distributed over the many grains present in an electrode, mainly due to the difficulty to probe the phase nucleation and growth within individual grains. This is crucial information for future battery improvement because the local conditions, such as the local current density, govern the Li-ion battery cycle life and rate performance. Using the microbeam at ID11, operando X-ray diffraction was employed to monitor the transformation of many individual LiFePO4 particles inside an Li-ion battery electrode from slow to fast battery (dis)charging rates, as shown schematically in Figure 26a. The diffraction results revealed the cycling-rate dependent phase transition mechanism, shown in Figure 27, within individual electrode grains. Measuring the transformation process of individual LiFePO4 particles allowed the first quantification of local current densities, i.e. the current per surface area of individual grains.
 |
|
Fig. 26: (a) Schematic representation of the in situ synchrotron X-ray diffraction experiment. (b) Charging voltage curve including the evolution of a 2D (200) LiFePO4 (LFP) and (200) FePO4 (FP) peak showing the progressive FP formation and LFP disappearance. |
Due to the sub-micrometre sized and intense X-ray beam, the diffraction rings that are observed from powder diffraction break up into individual spots, each representing an individual nanosized LiFePO4 grain. This allowed the evolution of the (200) reflection to be monitored for individual LiFePO4 particles during charge and discharge, as shown in Figure 26. The gradual disappearance of the (200) LiFePO4 and appearance of the (200) FePO4 diffraction peaks reflect the first-order phase transition when Li-ions are removed from the LiFePO4 electrode.
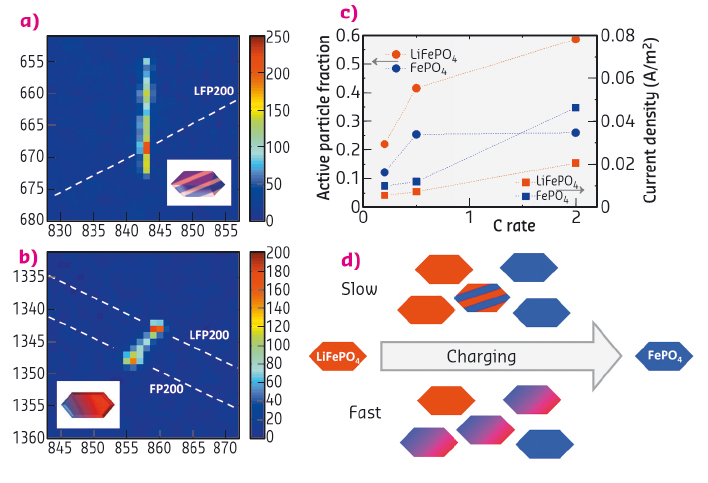
A surprising observation is that a significant fraction of the reflections during slow charge and discharge appear as streaks as illustrated by the reflections in Figure 27a, rather than symmetric peaks as shown in Figure 26b. The considerable one dimensional broadening implies platelet-shaped domains of both FePO4 and LiFePO4 as shown in Figure 27a, where the plate thickness is inversely proportional to the length of the streaks.
When the charge and discharge rate is increased, the streaked reflections narrow and disappear, translating into a decrease in number of platelet domains and an average increase in platelet domain thickness. At fast charging, a double peaked reflection appears, Figure 27b, where the appreciable intensity between the LiFePO4 (200) and FePO4 (200) end-member indicates a continuous distribution of intermediate compositions, reflecting a diffuse interface over the full length scale of the 140 nm grain. This proves that the recently observed bypassing of the first-order phase transition [1] occurs within a single grain, rationalising the excellent battery performance of this electrode material.
 |
|
Fig. 27: Phase transformation mechanism slow and fast cycling rates: (a) For a slow charge rate vertical (200) streaks are observed for both LiFePO4 (LFP) and FePO4 (FP) phases. The broadening in the z-direction gives the thickness of the platelet domains in this case leading to 10 nm in 140 nm particles. This leads to the shown internal two phase FP/LFP morphology. (b) (200) reflection at a high charging rate showing coexistence of the LFP and FP phases within a single grain. The dashed lines indicate the powder rings for the (200) reflections of the LFP and FP phases, indicated as LFP200 and FP200, respectively. For both phases the a-axis is closely aligned indicating a diffuse coherent interface. (c) Active fraction and current density of the particles resulting from the average transformation times where C/n denotes the rate at which a full charge or discharge takes n hours. (d) Sketch of the rate-dependent transformation upon charge as follows from the microbeam diffraction experiments (similar upon discharge only with a smaller active particle fraction, see (c)). |
Measuring the transformation process of individual LiFePO4 particles allowed the first quantification of local current densities in Li-ion battery electrodes under realistic charge and discharge conditions, as shown in Figure 27c. The local current densities are larger than expected if all electrode active material is assumed to participate but smaller than that expected from the established particle-by-particle mosaic transformation. The derived charge and discharge rate dependence of the local current density is a crucial parameter for electrode design that can help to prevent the occurrence of hotspots and fractures in electrodes.
Principal publication and authors
Direct view on the phase evolution in individual LiFePO4 nanoparticles during Li-ion battery cycling, X. Zhang (a), M. van Hulzen (a), D.P. Singh (a), A. Brownrigg (b), J.P. Wright (b), N.H. van Dijk (a) and M. Wagemaker (a), Nature Communications 6 (2015); doi: 10.1038/ncomms9333.
(a) Department of Radiation Science and Technology, Delft University of Technology (The Netherlands)
(b) ESRF
References
[1] X. Zhang et al., Nano Letters 14, 2279-2285 (2014).



