- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Electronic structure, magnetism and dynamics
- Understanding magnetic coupling in lanthanide-based molecules by XMCD and ab initio modelling
Understanding magnetic coupling in lanthanide-based molecules by XMCD and ab initio modelling
A comprehension of the mechanisms linking a molecular spin centre with its environment is crucial for the development of spintronics functionalities at the molecular scale. Here we combine X-ray magnetic circular dichroism (XMCD) with ab initio calculations to investigate the microscopic spin path in bis(phthalocyaninato)-lanthanide(III) single molecule magnets coupled to a Ni substrate.
Magnetic molecules of the family of bis(phthalocyaninato)-lanthanide(III) (LnPc2, so called "double decker") are made by a single lanthanide ion as the magnetic centre, sandwiched between two phthalocyanines (Figure 9, inset). This specific structure, where the localised lanthanide f-orbitals are coupled with the delocalised p-type electrons of the organic ligands, allows the existence of a sizeable magnetic interaction with the environment, while at the same time the magnetic core is efficiently protected. The intramolecular spin communication channels are highlighted here by studying how the coupling between the molecules and a Ni substrate is varied upon the substitution of the central Ln-ion.
 |
|
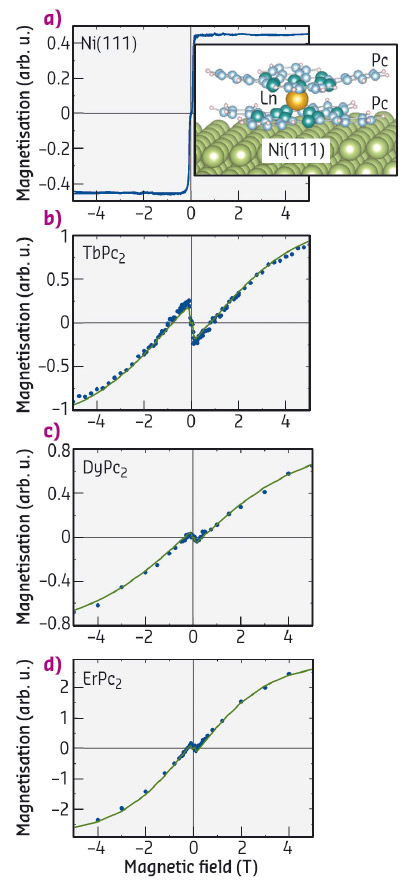
Fig. 9: Element resolved XMCD magnetisation measurements of Ni (a), Tb (b), Dy (c), Er (d) for the LnPc2/Ni(111) systems, taken at the incidence angle Θ = 70° between the X-ray beam and the surface. Experimental data are shown as dots, while the theoretical fit are the continuous lines. |
Our experiments were carried out at beamline ID08 (now ID32). The molecular units were sublimated in situ on a freshly prepared Ni(111) surface with sub-monolayer coverage. Our results show that they are isolated and lying flat, with the Pc plane parallel to the surface. The low-temperature (T = 8 K) XMCD-derived magnetisation curves of the three compounds (LnPc2, where Ln = Tb, Dy and Er) and of the Ni substrate are shown in Figure 9. The Ln-curves display a characteristic "N-shape" behaviour, in particular for small magnetic fields where the magnetisation is generally opposed to the field. These features reveal an antiferromagnetic coupling of the Ln-magnetic moment with the Ni substrate. The quantitative estimation of the coupling strength can be obtained by fitting the experimental data with a model spin-Hamiltonian, where the only fitting parameter is the Ln-Ni coupling and all the microscopic molecular details are estimated by ab initio methods. The results are the continuous lines in Figure 9, while the values for the coupling strength are plotted in Figure 10b, showing a clear dependence of the interaction with the Ln ion, and indicating the key role of the Ln orbital wave function in determining the magnetic couplings.
 |
|
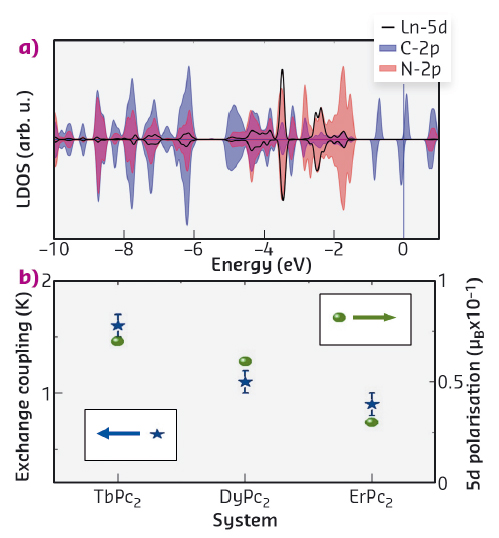
Fig. 10: a) Spin-resolved LDOS projected on the 5d orbital of Ln (very similar in all three cases) and on the 2p orbitals of the nearest-neighbours (to the Ln ions) N and C atoms in the phthalocyanines. b) Magnetic coupling strength, as determined from the fit (left axis), and magnitude of the magnetic polarisation (right axis) induced on the 5d orbitals for each Ln compound calculated from DFT. |
Deeper insights into the intramolecular interaction are obtained by the DFT calculations of the molecular compounds. Our analysis reveals a partial occupation, together with a finite spin polarisation, of the lanthanide 5d orbitals induced by the localised 4f electrons that carry the magnetic character of the Ln ion. The values of the induced spin polarisation depend on the specific Ln atom and are shown in Figure 10b, displaying the same trend in going from Tb to Er as observed experimentally for the magnetic coupling strengths. The Ln 5d electrons, in turn, overlap and hybridise with the p-states of the C and N atoms in the phthalocyanines (Figure 10a). We can therefore conclude that such d-mediated interaction is the mechanism through which the spin information in the f-orbitals propagates to the delocalised states of the two phthalocyanines that finally communicate with the external world by direct contact (with the Ni magnetic surface in this case).
By a combined experimental and theoretical approach, the microscopic spin communication channels are identified in the prototypical case of LnPc2 magnetic molecules coupled to magnetic substrates. The most important feature is that there is not a direct overlap between the magnetic, localised 4f states and the Pc unit, but rather the interaction occurs through the auxiliary 5d orbitals of the Ln ion. This three-step spin communication channel [(4f) Ln ↔ (5d) Ln ↔ (Pc) ↔ surface] is responsible for the experimentally observed coupling between the Ln ions and their environment, yet preserving unaffected the pristine magnetic properties of the molecules.
Principal publication and authors
Spin-communication channels between Ln(III) bis-phthalocyanines molecular nanomagnets and a magnetic substrate, A. Candini (a), D. Klar (b), S. Marocchi (a), V. Corradini (a), R. Biagi (a,c), V. De Renzi (a,c), U. del Pennino (a,c), F. Troiani (a), V. Bellini (a), S. Klyatskaya (d), M. Ruben (d,e), K. Kummer (f), N.B. Brookes (f), H. Huang (g), A. Soncini (g), H. Wende (b) and M. Affronte (a,c), Scientific Reports 6, 21740 (2016); doi: 10.1038/srep21740.
(a) Centro S3, Istituto Nanoscienze - CNR, Modena (Italy).
(b) Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Duisburg (Germany)
(c) Dipartimento di Scienze Fisiche, Informatiche e Matematiche, Università di Modena e Reggio Emilia, Modena (Italy)
(d) Institute of Nanotechnology, Karlsruhe Institute of Technology (KIT), Eggenstein-Leopoldshafen (Germany)
(e) Institut de Physique et Chimie des Matériaux de Strasbourg (France)
(f) ESRF
(g) School of Chemistry, The University of Melbourne (Australia)



