- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Matter at extremes
- High pressure synthesis of 1D polymer/zeolite nanocomposites
High pressure synthesis of 1D polymer/zeolite nanocomposites
High pressure (GPa) synthesis of polyacetylene and polycarbonyl has been performed in the 1D channels of a pure SiO2 zeolite, thereby obtaining nanocomposites, which are good candidates as highly-directional semiconductors and high energy density materials, respectively.
Recently, simple carbon-based polymers have been synthesised at high pressures (GPa) in silicalite, a pure SiO2, non catalytic zeolite with a 3D system of mutually interconnected microchannels [1-3]. Pressure and confinement of the reacting monomers at the angstrom scale are the driving forces of the synthesis. These protocols permitted otherwise unstable polymers to be stabilised and protected from the atmosphere, and to obtain an entirely novel class of nanocomposites with modified physical properties. Unfortunately, channel interconnection in silicalite prevents ideal, isolated polymer chains from being obtained. In this work, we overcame these limitations by using a non-catalytic, all SiO2 zeolite, ZSM-22 (TON), with a 1D channel system as the host material, which could permit the exploitation of the directional properties of the polymers. In this zeolite, we have synthesised polyacetylene (PA) and polycarbonyl (pCO), two archetypal, linear polymers, a 1D semiconductor and a high energy density polymer, respectively. These two nanocomposites were named PA/TON and pCO/TON.
 |
|
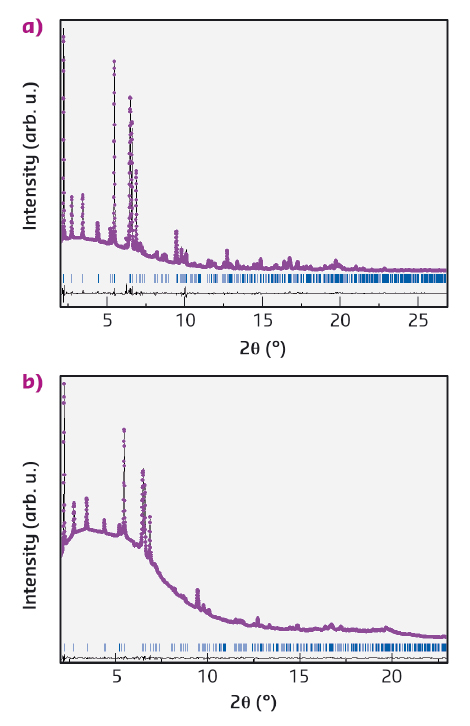
Fig. 124: Experimental (dots) and calculated (solid line) XRD profiles from the Rietveld refinement of PA/TON (a) and pCO/TON (b) recovered at ambient pressure. Wavelengths: 0.414132 Å for pCO/TON, and 0.415352 Å for PA/TON. |
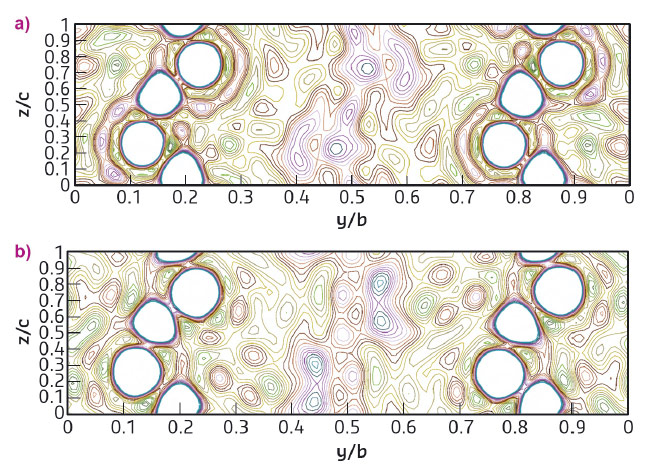
For the synthesis, acetylene and carbon monoxide were cryogenically loaded into diamond anvil cells in the solid and the liquid phase, respectively, together with hydrophobic TON powder. IR absorption spectra of the reacting mixtures at pressures up to 10 GPa and of nanocomposites recovered under ambient conditions revealed chemical signatures of the embedded polymers such as the C-H stretching peak for C in planar, sp2 hybridisation in PA/TON, and the C=O stretching peak in pCO/TON. Synchrotron powder X-ray diffraction (XRD) patterns of the recovered samples were measured at beamline ID09A (now ID15B) and then refined with the Rietveld method. Very high quality data and structural refinement of the SiO2 framework and of embedded C atoms were obtained for PA/TON, as reported in Figures 124a and 125a. The PA content is two chains per pore with 50% occupancy, which is consistent with the presence of a degree of disorder in the chain isomers and positions and corresponds to 100% pore filling. The PA chains have a planar zigzag conformation parallel to the bc plane (Figure 125a), and density functional theory (DFT) calculations indicate that the cis-PA conformer may dominate. A very good fit to the XRD patterns of pCO/TON was also obtained from the Rietveld refinement, Figure 124b. The refined occupations give a CO content of 2.4 molecules per unit cell corresponding to 30% pore filling in a statistical distribution. Incomplete pore filling is probably linked either to an incomplete polymerisation of CO and subsequent loss of remaining monomers after pressure release or to incomplete filling by the initial CO monomers. The structural model consists of alternating cis and transoid CO configurations lying in the bc planes, which are commensurate with the framework and consistent with the observed Fourier map, Figure 125b, as also shown by DFT calculations. In addition, DFT calculations of the electronic density-of-states of ordered pCO/TON and PA/TON show van Hove singularities within the band gap of the TON due to the 1-D nature of the polymer chains.
 |
|
Fig. 125: Section of the electron density map at x/a = 0.5. The white circles represent the framework atoms, whereas the polymerised chains are visible at y/b = 0.4-0.6 for PA/TON (a) and pCO/TON (b). |
Confined PA and pCO in the nanocomposites are chemically ordered, with some isomeric disorder in PA, in contrast to bulk PA and pCO. pCO/TON is a material with an energy storage capacity higher than that of dynamite. The protecting TON allows this energy to be retained/stored in the presence of atmospheric moisture. In PA/TON, where TON also prevents PA from reacting with moisture, the 1D nature of the polymers may lead to the use of this class of materials as quantum devices for important applications such as quantum computation.
Principal publication and authors
M. Santoro (a,b), D. Scelta (b,c), K. Dziubek (b,d), M. Ceppatelli (c,b), F.A. Gorelli (a,b), R. Bini (b,e), G. Garbarino (f), J.-M. Thibaud (g), F. Di Renzo (g), O. Cambon (g), P. Hermet (g), J. Rouquette (g), A. van der Lee (h) and J. Haines (g), Chem. Mater. 28, 4065 (2016); doi: 10.1021/acs.chemmater.6b01639.
(a) CNR-INO, Sesto Fiorentino (Italy)
(b) LENS, Sesto Fiorentino (Italy)
(c) CNR-ICCOM, Sesto Fiorentino (Italy)
(d) Faculty of Chemistry, Adam Mickiewicz University, Poznań (Poland)
(e) Chemistry Department, University of Florence, Sesto Fiorentino (Italy)
(f) ESRF
(g) ICGM-CNRS, and Université de Montpellier (France)
(h) IEMM-CNRS, and Université de Montpellier (France)
References
[1] M. Santoro et al., Nat. Commun. 4, 1557 (2013).
[2] D. Scelta at al., Chem. Mater. 26, 2249 (2014).
[3] M. Santoro et al., Chem. Mater., 27, 6486 (2015).



