- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Matter at extremes
- Revealing the molecular structure of kerogen in gas shale
Revealing the molecular structure of kerogen in gas shale
Fracking is a controversial recovery method to extract oil or gas from shale rock. Little is known about the microscopic structure of kerogen, the organic matter which hosts hydrocarbons in gas shales. Using a hybrid strategy, combining molecular simulation and experiment, we have developed molecular models of kerogen which capture the complexity of such geological structures. These atom-scale structures, which have been derived and validated against experimentally-determined properties, can be used to unravel the behaviour of this complex organic matter.
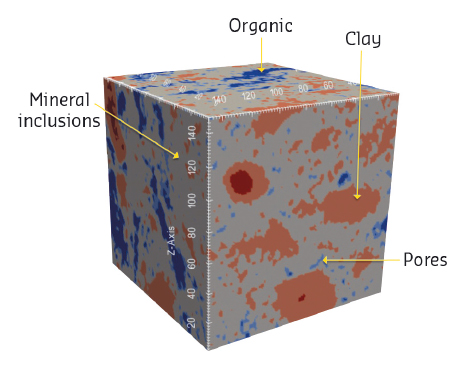
Oil and natural gas are produced from the decomposition of organic matter, known as kerogen, in shale rocks. In these structures, the kerogen is embedded within a mineral mixture (Figure 133) resulting in permeabilities a million times lower than in conventional hydrocarbon reservoirs. Owing to such ultra-low permeability combined with fast, unexplained productivity decline, the hydrocarbons are recovered through a controversial extraction technique known as hydraulic fracturing, or fracking. To develop new techniques that would be both effective and lower in environmental impact, one needs a better understanding of the shale structure in general and the kerogen structure in particular.
 |
|
Fig. 133: X-ray microscopy image of an untreated sample of gas shale, showing inclusions of clay, organic matter and other minerals. Copyright: M. Hubler (MIT) and J. Gelb (Carl Zeiss X-ray Microscopy). |
We have studied four different kerogen samples of different geological origins and degrees of maturation. Their atomic structure, texture, density and mechanical properties were characterised thanks to beamlines ID11 and ID27. At ID11, we measured the pair distribution functions that were later used in our atomistic reconstruction procedure. At ID27, the sample was placed in a diamond anvil cell with varying pressures of up to 5.1 GPa in order to measure its bulk modulus. We have also used inelastic neutron scattering and neutron diffraction at Oak Ridge National Laboratory in the USA in order to have a complete set of structural and vibrational information about our samples.
 |
|
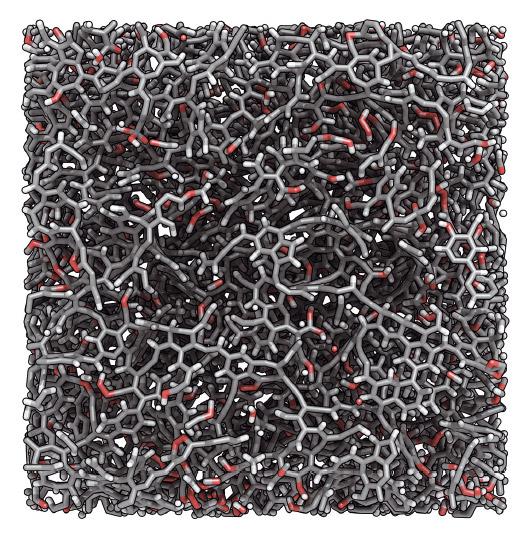
Fig. 134: Molecular model of a sample of the Marcellus kerogen studied (the organic phase constitutes the source of hydrocarbons in shale gas). Carbon, hydrogen and oxygen atoms are shown in grey, white and red respectively. The size of the image is 5 × 5 nm. Four samples with different maturities, that is, with different extents of thermal alteration in the subsurface, were considered. Copyright: C. Bousige (CNRS). |
Using a hybrid method that combines experimental structural constraints and Monte Carlo minimisation (the so-called Hybrid Reverse Monte Carlo method), we developed molecular models of kerogen with different maturities based on their chemical composition, texture and density (Figure 134). These atomistic models were then validated by comparing them to experimentally accessible kerogen properties, such as bulk moduli, vibrational densities of states, pore size distributions, etc. Once the models are validated, they can be used to study properties of kerogen that are otherwise difficult to access experimentally. For example, we showed that kerogen’s maturation, which manifests itself as an increase in the sp2/sp3 hybridisation ratio, entails a crossover from plastic to brittle rupture mechanisms.
In brief, these molecular models are a fundamental building block that allows the adsorption, mechanical, and transport properties to be unravelled for this complex organic matter known as kerogen. These results can be used in up-scaling strategies such as lattice methods in order to bridge the gap in a “bottom-up” fashion between the atomic and the engineering scales.
Principal publication and authors
Realistic molecular model of kerogen’s nanostructure, C. Bousige (a,b), C. Matei Ghimbeu (c), C. Vix-Guterl (c), A.E. Pomerantz (d), A. Suleimenova (d), G. Vaughan (e), G. Garbarino (e), M. Feygenson (f), C. Wildgruber (f), F.-J. Ulm (a,b), R.J.-M. Pellenq (a,b,g) and B. Coasne (a), Nature Materials 15, 576–582 (2016); doi: 10.1038/nmat4541.
(a) Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge (USA)
(b) <MSE>2, UMI 3466 CNRS-MIT, Cambridge (USA)
(c) Institut de Science des Matériaux de Mulhouse (IS2M), UMR 7360 CNRS – UHA, Mulhouse (France)
(d) Schlumberger-Doll Research, Cambridge (USA)
(e) ESRF
(f) Oak Ridge National Laboratory (USA)
(g) CINaM, CNRS/Aix Marseille Université, Marseille (France)



