- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structural biology
- Structural basis for norovirus inhibition by human milk oligosaccharides
Structural basis for norovirus inhibition by human milk oligosaccharides
Human noroviruses bind histo-blood group antigens (HBGAs) and human milk oligosaccharides (HMOs) at an identical pocket on the virus particles. However, HBGAs are thought to enhance norovirus infections, whereas HMOs may inhibit infections. Structurally, HBGAs and HMOs are similar, but functionally, these "sugars" may act differently.
Human noroviruses are the dominant cause of acute outbreaks of gastroenteritis. Noroviruses are known to interact with oligosaccharides, termed histo-blood group antigens (HBGAs). This interaction is thought to be important for norovirus infections. HBGAs are found in saliva and are also expressed on the mucosal epithelium of the digestive tract. In comparison, human milk oligosaccharides (HMOs), the third most abundant component of human breast milk, also contain similar building blocks to HBGAs. HMOs are thought to act as a “receptor decoy” for certain pathogens. Indeed, one study found that human milk might provide some protection to infants with a norovirus infection [1].
To better understand norovirus binding interactions with these oligosaccharides from a structural perspective, we solved X-ray crystal structures of the norovirus protruding protein (P domain) in complex with HMOs, i.e., 2’-fucosyllactose (2’FL) and 3-fucosyllactose (3FL). We also showed that these HMO’s could block norovirus from binding to HBGA.
 |
|
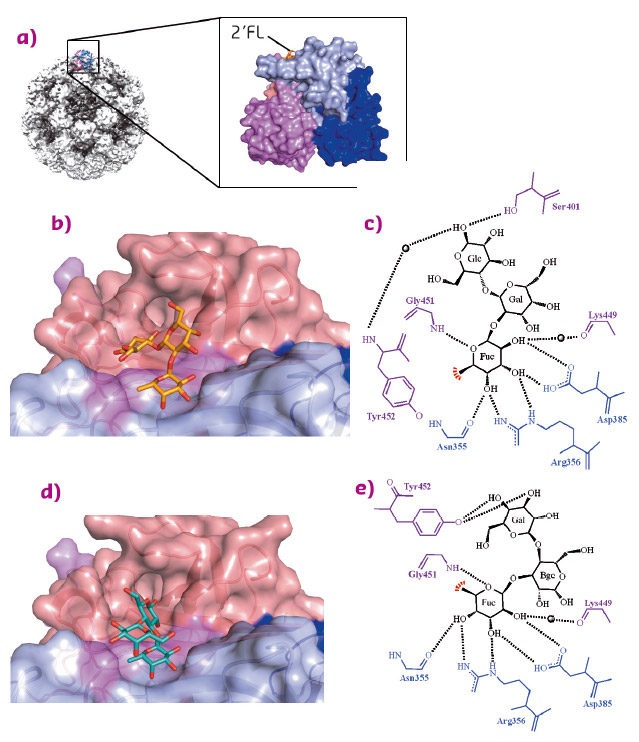
Fig. 88: HBGA and HMO interactions with norovirus. a) Cryo-EM structure of a norovirus VLP. b) The crystal structure of the norovirus P domain-2'FL complex determined to 1.55 Å resolution and coloured according to monomers (chain A and B) and P1 and P2 subdomains, i.e. chain A: P1 (blue), chain A: P2 (lightblue), chain B: P1 (violet), and chain B: P2 (salmon). c) P domain interactions with 2'FL, showing α-fucose (Fuc), α-galactose (Gal), and β-glucose (Glc). The black lines represent hydrogen bonds and the red fan shows the hydrophobic interaction with Tyr452. Black spheres represent water molecules. d) The crystal structure of the norovirus P domain-3FL complex determined to 1.35 Å resolution. e) P domain interactions with 3FL showing α-fucose (Fuc), β-galactose (Bgc), and β-glucose (Gal). |
HBGAs bind at the top of the norovirus P domain and both 2’FL and 3FL bind at the pocket on the norovirus capsid (Figure 88a). The HMOs are held in place by a network of hydrophilic and hydrophobic interactions at the P domain dimeric interface (Figures 88b to 88e). The fucose moiety of HMOs is held by a common set of conserved residues that also held the fucose of HBGAs. The central saccharides of HMOs are poorly held by the protein, while the terminal saccharides interact with varying residues. Overall, the HMO binding interactions were comparable to HBGA interactions [2,3].
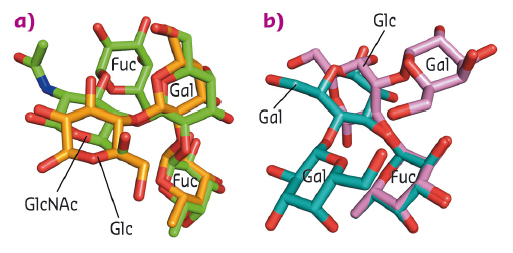
Our structural data showed that both HBGAs and HMOs bind in copious orientations. However, the fucose moiety of HBGAs and HMOs is always held in an identical position, whereas the other saccharide units are positioned differently (Figure 89). Terminal saccharide units are usually only held with few if any direct hydrogen bonds, suggesting a weaker interaction and likely allowing for the numerous oligosaccharide positions observed.
 |
|
Fig. 89: HMO inhibition of the HBGA binding pocket. a) Superposition of 2'FL and Lewis-Y tetrasaccharide (green sticks) show that the 2'FL saccharide units mimick the orientations of the first three saccharides of Lewis-Y. b) Superposition of 3FL and B-trisaccharide (pink sticks) indicate that the fucose units are similarly positioned, whereas the other saccharide units are orientated differently. |
To describe a possible biological function for the HMOs, we performed a HMO inhibition study with norovirus particles and HBGAs. We found that the HMOs were able to block norovirus particles from binding to HBGAs in a mostly dose dependent manner. The half maximal inhibitory concentration (IC50) in the HBGA assays for 2’FL and 3FL were 5.5 mM and 5.6 mM, respectively. These results showed that HMOs might block the HBGA pocket and, considering that mother’s milk contains ~1-5 mM of 2’FL/3FL, might function as weak norovirus antivirals.
In summary, we showed that the norovirus HBGA/HMO binding site is a multipurpose oligosaccharide pocket. 2’FL and 3FL may act as natural decoys in an infection by mimicking the structure of the HBGAs and binding at the HBGA pocket. Currently, there is no treatment or vaccine available for human norovirus infections, which cause a massive burden of disease worldwide. Importantly, 2’FL has already been shown as a safe food supplement for infant formula [4]. Further clinical studies are planned with 2’FL and 3FL, as well as with more complex HMO-structures.
Principal publication and authors
Structural basis for norovirus inhibition by human milk oligosaccharides, S. Weichert (a), A. Koromyslova (b,c), B.K. Singh (b,c), S. Hansman (a,b), S. Jennewein (d), H. Schroten (a) and G.S. Hansman (b,c), J Virol 90, 4843–4848 (2016); doi: 10.1128/JVI.03223-15.
(a) Pediatric Infectious Diseases Unit, University Children’s Hospital Mannheim, University of Heidelberg, Mannheim (Germany)
(b) Schaller Research Group at the University of Heidelberg and the DKFZ, Heidelberg (Germany)
(c) Department of Infectious Diseases, Virology, University of Heidelberg (Germany)
(d) Jennewein Biotechnology, Rheinbreitbach (Germany)
References
[1] A.L. Morrow et al., J Pediatr 145, 297-303 (2004).
[2] G.S. Hansman et al., J Virol 85, 6687-6701 (2011).
[3] B.K. Singh et al., J Virol 89, 2024-2040 (2015).
[4] M. Coulet et al., Regul Toxicol Pharmacol 68, 59-69 (2014).



