- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Electronic structure, magnetism and dynamics
- Human scalp hair records the source of mercury exposure
Human scalp hair records the source of mercury exposure
HR-XANES spectroscopy was used to decipher the molecular forms of mercury in human tissue from individuals exposed inadvertently to mercury contamination. The low-Z atoms around Hg in scalp hair were identified, revealing the source of mercury exposure. In combination with nano-imaging, the timing of an exposure event was discovered.
Common sources of mercury that affect human health are methylmercury (MeHg) in fish and rice, ethylmercury (EtHg) in medical products and elemental mercury in dental amalgams (Hg0), and mercury vapour in occupational areas. The hypothesis that the molecular forms of mercury in human hair could be an indicator of the source of the mercury was tested. Scalp hair is convenient for this purpose because it typically contains 250 times more mercury than blood, is easy to sample, and provides a history of exposure with a daily growth rate of about 40 micrometres. Mercury has a low natural abundance, at most 10 ppm (ng/mg) in the hair of fish consumers and at the ppm to sub-ppm level in the general population. Another impediment to assessing the form of mercury in hair is the proteinaceous composition of this material, therefore requiring the use of probes that can differentiate C, N, and O ligands (Figure 89a).
 |
|
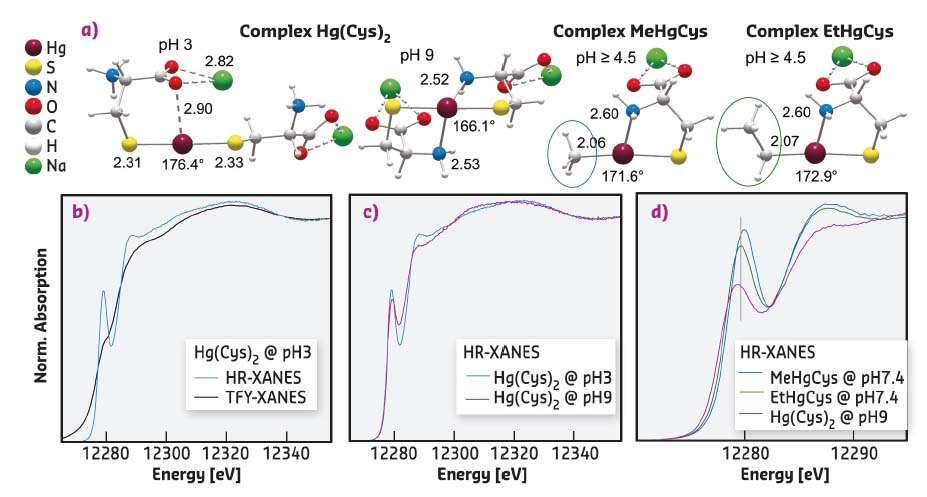
Fig. 89: a) Molecular structures of Hg-thiolate complexes optimised by MP2/TZVP-ecp calculations. Interatomic distances are in Ångstroms and S-Hg-S angle in degrees. b-d) Hg L3-edge HR-XANES signatures of the Hg-thiolate complexes. |
To tackle both detection limit and sensitivity to low-Z ligands, high-energy-resolution XANES spectroscopy was used at beamline ID26. HR-XANES has higher signal-to-noise (s/n) ratio than EXAFS, and also possesses better chemical-state and ligand sensitivity because of participation of unoccupied valence orbitals in the excited state [1]. This sensitivity was enhanced by measuring the X-ray fluorescence with analyser crystals in order to reduce the intrinsic broadening of the spectral features [2].
At the Hg L3-edge of the measurements, HR-XANES had an effective energy resolution of 3.0 eV compared to about 6.1 eV in conventional total fluorescence yield (TFY) measurement with a solid-state detector. The gain in spectral resolution is shown in Figure 89b with the 2p3/2 → 6s/5d electronic transition of the bis L-cysteinate complex (Hg(Cys)2) at pH 3. The linear dithiolate conformation gives a shoulder in TFY measurement and a prominent near-edge peak in HR-XANES measurement.
The whole rising region of HR-XANES contains core-to-valence features that vary in intensity and energy with the number and identity of atoms in the first and second coordination shell of Hg, and with the geometry of the bonding environment. For instance, secondary amine ligands, which bond at pH 9, give a distinctive spectral signature characterised by a less intense near-edge peak and a shift to lower energy of the HR-XANES trace beyond this peak (Figure 89c). Also, Hg(Cys)2, MeHgCys and EtHgCys complexes are readily differentiated from the energy of the 2p3/2 → 6s/5d transition (Figure 89d).
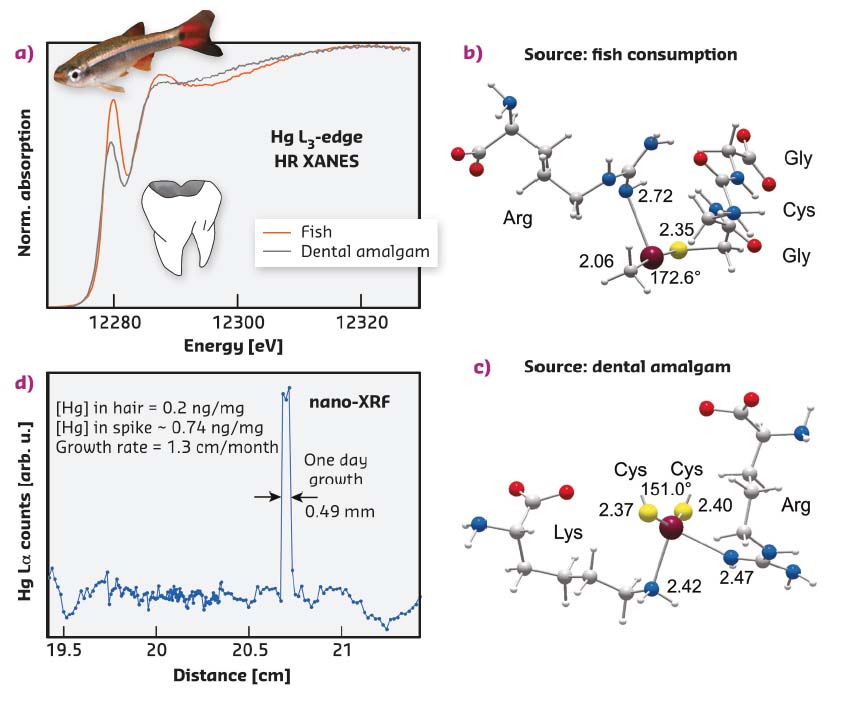
Mercury sourced from fish consumption and dental amalgam gives diagnostic HR-XANES features (Figure 90a) that were used to model its bonding environment in hair using first principles calculations. Figure 90b shows the optimised geometry of a MeHg[SR+N] coordination complex with a cysteinyl sulfur from a GlyCysGly tripeptide and a side chain guanidyl NH donor from arginine, consistent with the HR-XANES results for organic methylmercury in hair. The bending of the linear Me-Hg-SR coordination from about 180° to 172.6° constitutes a noteworthy structural marker of the Hg-N secondary bond (either through a NH or NH2 group), which gives a distinctive XANES signature.
 |
|
Fig. 90: a) HR-XANES signatures of two chemical forms of mercury in hair, one originating from fish consumption (MeHg) and the other from removal of a dental amalgam (Hg0). b,c) Geometry-optimised models representing the two types of bonding environments in hair proteins (MP2/TZVP-ecp optimisation). d) Longitudinal profile of methylmercury in a single strand from an individual affected by extraction of a dental amalgam. |
Mercury sourced from dental amalgams is inorganic and the Hg(II) ion coordinated to two thiolate ligands (SR-) and to two secondary N ligands in a seesaw geometry (Figure 90c). The timing of a particular exposure event resulting from the removal of an amalgam was determined by coupling HR-XANES at ID26 with Hg nano-imaging at ID16B. The mercury spike on the longitudinal Hg(Lα) profile from a single strand of hair (Figure 90d) recorded the contamination event, and its width indicates the residence time of Hg(II) in the bloodstream, here estimated to be about one day. The dental source of Hg(II) was revealed by its Hg[(SR)2+N2] coordination in the spike area, as determined by HR-XANES.
Principal publication and authors
Chemical forms of mercury in human hair reveal sources of exposure, A. Manceau (a), M. Enescu (b), A. Simionovici (a), M. Lanson (a), M. Gonzalez-Rey (c), M. Rovezzi (d), R. Tucoulou (d), P. Glatzel (d), K.L. Nagy (e) and J-P. Bourdineaud (f), Environmental Science & Technology 50, 10721-10729 (2016); doi: 10.1021/acs.est.6b03468.
(a) ISTerre, Université Grenoble Alpes, CNRS, Grenoble (France)
(b) Laboratoire Chrono Environnement, Université de Franche-Comté, CNRS, Besançon (France)
(c) Laboratoire EPOC, Université de Bordeaux, CNRS, Arcachon (France)
(d) ESRF
(e) Department of Earth and Environmental Sciences, University of Illinois at Chicago (USA)
(f) Institut Européen de Chimie et Biologie, Université de Bordeaux, CNRS, Pessac (France)
References
[1] A. Manceau et al., Inorg. Chem. 54, 11776-11791 (2015).
[2] P. Glatzel et al., J. Elec. Spec. Relat. Phenom. 188, 17-25 (2013).



